Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.72 no.10 Johannesburg Nov. 2017
http://dx.doi.org/10.17159/2519-0105/2017/v72no10a4
CLINICAL REVIEW
Fragmentary tooth root development: biological and forensic dental implications
S NelI; CL DavidsonII; A UysIII; L SykesIV; H BernitzV
IBChD, MSc. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, Pretoria
IIBChD, MSc. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, Pretoria
IIIBSc, BChD, MSc. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, Pretoria
IVBSc, BDS, MDent (Pros). Department of Prosthodontics, School of Dentistry, Faculty of Health Sciences, University of Pretoria
VBChD, MSc, PhD. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, Pretoria
ABSTRACT
Recent findings indicate that there could be continued root development after the successful surgical removal of an impacted tooth. The paper provides a brief review of normal root development, emphasizing the chain of reciprocal epithelial-ectomesenchymal interactions which regulate all aspects of this process.
Mineralized dental structures are not an absolute requirement for tooth root development, but residual fragments of Hertwig's epithelial root sheath (HERS), together with the associated ectomesenchymal cells, will enable continued growth. The findings presented in this paper have significant implications in forensic odontology, dental litigation and for routine and elective tooth extractions.
INTRODUCTION
In 2015, during the routine forensic identification of a mutilated corpse, a peri-apical radiograph revealed a sizeable residual root in the 38 area (Figure 1). The ante-mortem records supplied by an orthodontist consisted of a panoramic radiograph, taken of the deceased in December of 2009 (Figure 2).

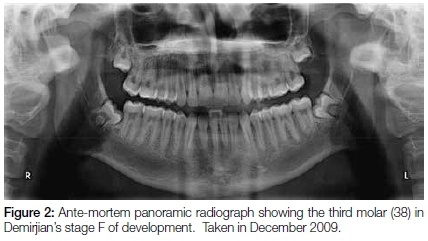
A maxillo-facial surgeon subsequently used this very radiograph four months later in March 2010 during surgery to remove the wisdom teeth for elective orthodontic purposes.
The ante-mortem panoramic radiograph clearly shows the root formation of the tooth 38 as Demirjian's stage F with root length as great as the crown length (Figure 2).1
The peri-apical radiograph taken during the post-mortem investigation was inconsistent with this ante-mortem record as it revealed a horizontally positioned residual root with a closed apex in the 38 area and both the lamina dura and periodontal ligament space were not only present but extended around the entire root surface (Figure 1). Unfortunately, there were no post-extraction radiographs available, nor documented records indicating whether any of the roots had fractured during the extraction procedure. However, it would not have been possible for the 38 to have completed root development as seen in Figure 1 in the four months between the taking of the panoramic radiograph and the extraction of the wisdom tooth. The presence of the developed root created a forensic dilemma, as it constituted an apparently inexplicable discrepancy despite there being several other concordant dental features between the ante-mortem and post-mortem radiographs. However, the body was later positively identified by fingerprints as that of the orthodontic patient. The confirmation of identity by non-dental means implied that a residual root fragment had continued developing after the extraction of the tooth.
The hypotheses proposed: Remnants of the apical aspect of Hertwig's epithelial root sheath (HERS) and the associated ectomesenchymal cells remained behind, leading to continued formation of the root.
REVIEW OF TOOTH ROOT DEVELOPMENT
Tooth development is initiated and regulated by a cascade of reciprocal interactions between the dental epithelium and the associated ectomesenchyme.2 The earliest sign of tooth development is regarded as a thickening of the odontogenic epithelium, wherein resides the initiating capacity to form teeth. As the dental ectomesenchyme condenses, this anlage is shifted to the underlying ectomesenchyme derived from the neural crest.3 Thereafter the reciprocal signalling between epithelial and ectomesenchymal cells continues through the characteristic bud, cap and bell stages of crown formation.4 This interaction is mediated through multiple pathways and a variety of different transcription factors.5-7 Details on the precise molecules involved are however beyond the scope of this article.
While the molecular and cellular mechanisms of early tooth development and crown morphogenesis have been extensively studied, less is known about the molecular mechanisms controlling tooth root formation.7,8 However, great progress has been made over the last ten years in this field.9 Root formation follows the completion of crown formation with the inner and outer enamel epithelium of the enamel organ forming a continuum at the cervical loop that extends apically as a thin sheath.10 This structure is known as Hertwig's epithelial root sheath (HERS).11 Morphologically, HERS forms a structural boundary between two dental ectomesenchymal tissues derived from neural crest cells, namely: the dental papilla and the dental follicle.9,10 HERS signals the ectomesenchymal cells of the dental papilla to differentiate into odontoblasts.10 The secretion of Laminin 5 and TGF-β by HERS seems to be crucial in this process. Laminin 5 appears to induce migration, growth and differentiation of the ectomesenchymal cells while TGF-β is believed to induce the differentiation of these cells into odontoblasts.9 TGFβ1 induces early odontoblast differentiation through the Smad signalling pathway whereas Nfic signalling modulates late odontoblast differentiation and mineralization.9 These newly differentiated odontoblasts then secrete predentine that will become mineralized root dentine.10 The epithelial component (i.e. HERS) is therefore crucial in initiating root formation and is responsible for guiding and determining the size, shape and number of roots.12-14 Any disturbance in HERS can result in irregularities in root development.15 If the continuity of HERS is disrupted prematurely, the odontoblasts fail to differentiate with no subsequent dentin or cementum formation.9
As soon as the odontoblasts have differentiated, HERS undergoes fragmentation through the degradation of E-cadherin, again under the influence of TGF-β1.16 Ectomesenchymal cells of the dental follicle then penetrate this bi-layer and deposit initial cementum.17 TGF-β1 signalling from HERS is responsible for inducing cementoblast differentiation.18 Some authors have postulated a different origin of cementoblasts where HERS cells themselves undergo epithelial-ectomesenchymal transformation and directly differentiate into cementoblasts.9,16,19 TGF-β1 and FGF2 have been proposed as factors stimulating this epithelial-ectomesenchymal transformation of HERS cells through a MAPK/ERK-dependent signalling pathway.20 Further support for this theory is that HERS has shown expression of cementoblast markers.13 However, this remains a controversial issue with recent evidence confirming the mesenchymal origin of cementoblasts.21
Current research suggests that there may be other systems and factors influencing root formation. Loss of the parathyroid hormone-related protein receptor (PTHrP-PPR) signalling in dental mesenchymal cells has been shown to alter the morphology of the roots and dysregulate cementum formation.21 Deletion of Sirtuin-6 (Sirt6) in mice exhibited stunted development of tooth roots as well a delay in tooth eruption.22
HYPOTHESIS REGARDING INDEPENDENT TOOTH ROOT FORMATION
In 1989, Thomas and Kollar demonstrated that HERS could induce odontoblast differentiation from the dental papilla.23 It is however important to note that this could take place only in papillae in which a certain degree of commitment already existed. Therefore, the dental papilla must have been exposed to signalling factors from HERS in order to be able to differentiate into odontoblasts. Based on this, had the apical aspect of HERS and the associated ectomesenchymal cells of the dental papilla and follicle been left intact in the case illustrated in this paper, that could account for the continued development of the tooth root.
The role of pre-programmed cells, as seen in stem cell studies, supports this hypothesis. Many investigators have used stem cells from dental tissues in the attempt to reconstruct a tooth that has normal physiological function.24-26 A 2006 study on miniature pigs used stem cells from the root apical papilla (SCAP) and periodontal ligament (PDLSCs) to construct a functional tooth root to which an artificial dental crown was fixed.27 The constructed root was successfully formed and functional, although the compressive strength of the bio-root was less than that of natural swine root dentin. SCAP can easily be isolated from the apical aspect of wisdom teeth in humans and show a greater tissue regeneration potential than do dental pulp stem cells (DPSCs).27 In the case presented, it is proposed that pre-programmed cells, SCAP and HERS, remained behind after the surgical procedure and retained the potential for continued root formation. The presence of a distinct periodontal ligament on the peri-apical radiograph surrounding the root (Figure 1) indicates that dental follicle cells must also have been present in order for this structure to develop.
Regenerative endodontic therapy for the treatment of immature non-vital teeth has similarly illustrated the functional advantages of viable HERS and SCAP in promoting further root formation and thickening of root dentin walls.28 In a recent study by Nazzal and Duggal on regenerative endodontics, the authors stress that although root development does occur, there is variability in the degree of success of these techniques. They continue by stating that preservation of structures like HERS will have a significant impact on the success of these treatments.29
The cascade of signalling events associated with the apical aspect of the developing tooth have not been completely elucidated.30 However, based on the availableexperimental data, we can hypothesize that a tooth root can continue to develop in the presence or absence of mineralized dental tissue. The presence of HERS with associated ectomesenchymal cells of the pulp and follicle remains crucial in order to maintain the epithelial-ectomesenchymal signalling cascade.
CLINICAL IMPLICATIONS
The dental identification of mutilated, decomposed and burned bodies relies on the comparison of ante-mortem and post-mortem dental records. An analysis of concordant features present may serve to either confirm or reject the identification of the mortal remains. This comparison involves all structures present in the dento-facial complex and can include: dental restorations, implant structures, tooth anatomy, sinus anatomy, dental anomalies, pathological lesions and any other recognisable features. The comparison of concordant features can be made with the aid of dental models, radiographs, constructed odontograms and hand written notes. Explicable discrepancies, as seen when radiographic angulations differ, are regularly observed and understood. However, the presence of a residual root, after the "complete" extraction of a particular tooth is more problematic. An undocumented residual root would constitute an inexplicable discrepancy and lead to a dental mis-match. The residual root in this case could only have been the result of continued growth of the root remnants left behind after the extraction of the tooth, which was at Demirjian's stage "F" root at the time of surgery. A thorough search of the literature was done and to the best of our knowledge, this is the first documented case of a residual root developing from residual tooth structures or cellular remains left behind during surgery. Forensic odontologists should be alerted to the fact that ante-mortem and post-mortem discrepancies of this nature are possible and explicable.
The discovery of a residual root after the removal of wisdom teeth by a maxillo-facial surgeon under general anaesthetic could most certainly lead to litigation by the unhappy patient if he/she is not informed of the possible complications. The findings of this paper will assist the defendant in cases of this nature, especially where due caution had been applied. The dental practitioner should carefully consider the forensic implications following routine extractions, elective extractions for orthodontic purposes and the surgical removal of impacted teeth. The importance of post-operative imaging to confirm complete extraction should be considered.
CONCLUSION
The evidence provided in the forensic case and consideration of the developmental biology, support the hypotheses that root formation could conceivably occur as long as pre-programmed cells for root formation are present, regardless of the presence or absence of mineralized dental tissues.
ACKNOWLEDGEMENTS
The authors would like to thank the South African Dental Association for the permission granted to include the x-ray images seen as Figures 1 and 2 in this paper. They formed part of an article on record- keeping published in the South African Dental Journal.31
ACRONYMS
DPSCs: dental pulp stem cells
ERK: extra-cellular signal-related kinases
FGF2: fibroblast growth factor
HERS: Hertwig's epithelial root sheath
MAPK: mitogen-activated protein kinases
PDLSCs: periodontal ligament stem cells
PTHrP-PPR: parathyroid hormone-related protein receptor
SCAP: stem cells from the root apical papilla
Sirt6: Sirtuin-6
References
1. Demirjian A, Goldstein H, Tanner JM. A new system of dental age assessment. Hum Biol. 1973;45(2):211-27. PubMed PMID: 4714564. [ Links ]
2. Hurmerinta K, Thesleff I. Ultrastructure of the epithelial-mesenchymal interface in the mouse tooth germ. Journal of Craniofacial Genetics and Developmental Biology. 1981;1(2):191-202. PubMed PMID: 7338550. [ Links ]
3. Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Archives of Oral Biology. 1987;32(2):123-7. PubMed PMID: 3478009. [ Links ]
4. Nanci A. Ten Cate's Oral Histology Development Structure and Function. 8th edition ed. St. Louis, Missouri: Elsevier; 2013.interactions regulating tooth development and renewal. [ Links ]
5. Current Topics in Developmental Biology. 2015;115:157-86. PubMed PMID: 26589925. [ Links ]
6. Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. Journal of Cell Science. 2003 ;1;116(Pt 9):1647-8. PubMed PMID: 12665545. [ Links ]
7. Li J, Parada C, Chai Y. Cellular and molecular mechanisms of tooth root development. Development. 2017 Feb 01;144(3):374-84. PubMed PMID: 28143844. Pubmed Central PMCID: PMC5341797. [ Links ]
8. Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, et al. beta-catenin is required in odontoblasts for tooth root formation. Journal of Dental Research. 2013 Mar;92(3):215-21. PubMed PMID: 23345535. [ Links ]
9. Huang XF, Chai Y. Molecular regulatory mechanism of tooth root development. International Journal of Oral Science. 2012 Dec;4(4):177-81. PubMed PMID: 23222990. Pubmed Central PMCID: 3633063. [ Links ]
10. Thomas HF. Root formation. The International Journal of Developmental Biology. 1995 Feb;39(1):231-7. PubMed PMID: 7626411. [ Links ]
11. Owens PD. Ultrastructure of Hertwig's epithelial root sheath during early root development in premolar teeth in dogs. Archives of Oral Biology. 1978;23(2):91-104. PubMed PMID: 274924. [ Links ]
12. Ten Cate AR. The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Diseases. 1996 Mar;2(1):55-62. PubMed PMID: 8957938. [ Links ]
13. Huang X, Bringas P, Jr., Slavkin HC, Chai Y. Fate of HERS during tooth root development. Developmental Biology. 2009 Oct 1;334(1):22-30. PubMed PMID: 19576204. Pubmed Central PMCID: 2744848. [ Links ]
14. Huang X, Xu X, Bringas P, Jr., Hung YP, Chai Y. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res. 2010 May;25(5):1167-78. PubMed PMID: 19888897. Pubmed Central PMCID: PMC3153373. [ Links ]
15. Luder HU. Malformations of the tooth root in humans. Front Physiol. 2015;6:307. PubMed PMID: 26578979. Pubmed Central PMCID: PMC4621611. [ Links ]
16. Itaya S, Oka K, Ogata K, Tamura S, Kira-Tatsuoka M, Fujiwara N, et al. Hertwig's epithelial root sheath cells contribute to formation of periodontal ligament through epithelial-mesenchymal transition by TGF-beta. Biomed Res. 2017;38(1):61-9. PubMed PMID: 28239033. [ Links ]
17. Diekwisch TG. The developmental biology of cementum. The International Journal of Developmental Biology. 2001 Sep;45(5-6):695-706. PubMed PMID: 11669371. [ Links ]
18. Farea M, Husein A, Halim AS, Berahim Z, Nurul AA, Mokhtar KI, et al. Cementoblastic lineage formation in the cross-talk between stem cells of human exfoliated deciduous teeth and epithelial rests of Malassez cells. Clinical Oral Investigations. 2015 Sep 22. PubMed PMID: 26392396. [ Links ]
19. Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen LS, Arzate H, et al. Role of Hertwig's epithelial root sheath cells in tooth root development. Developmental Dynamics : an official publication of the American Association of Anatomists. 2003 Dec;228(4):651-63. PubMed PMID: 14648842. [ Links ]
20. Chen J, Chen G, Yan Z, Guo Y, Yu M, Feng L, et al. TGF-beta1 and FGF2 stimulate the epithelial-mesenchymal transition of HERS cells through a MEK-dependent mechanism. Journal of Cellular Physiology. 2014 Nov;229(11):1647-59. PubMed PMID: 24610459. [ Links ]
21. Ono W, Sakagami N, Nishimori S, Ono N, Kronenberg HM. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat Commun. 2016 Apr 12;7:11277. PubMed PMID: 27068606. Pubmed Central PMCID: PMC4832076. [ Links ]
22. Liao X, Feng B, Zhang D, Liu P, Zhou X, Li R, et al. The Sirt6 gene: Does it play a role in tooth development? PloS one. 2017;12(3):e0174255. PubMed PMID: 28355287. Pubmed Central PMCID: PMC5371306. [ Links ]
23. Thomas HF, Kollar EJ. Differentiation of odontoblasts in grafted recombinants of murine epithelial root sheath and dental mesenchyme. Archives of Oral Biology. 1989;34(1):27-35. PubMed PMID: 2783039. [ Links ]
24. Oshima M, Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014 Jul;102(2):123-36. PubMed PMID: 25052182. [ Links ]
25. Yu T, Volponi AA, Babb R, An Z, Sharpe PT. Stem cells in tooth development, growth, repair, and regeneration. CurrentTopics in Developmental Biology. 2015;115:187-212. PubMed PMID: 26589926. [ Links ]
26. Yang L, Angelova Volponi A, Pang Y, Sharpe PT. Mesenchymal cell community effect in whole tooth bioengineering. Journal of Dental Research. 2017 Feb;96(2):186-91. PubMed PMID: 27927885. [ Links ]
27. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PloS One. 2006;1:e79. PubMed PMID: 17183711. Pubmed Central PMCID: 1762318. [ Links ]
28. EŞIan D, Monea AL. Morphological and developmentel characteristics of the Hertwig's epithelial root sheath and its involvement in the root growth and development of the immature teeth. Acta Medica Transilvanica. 2011;16(1):257-60. PubMed PMID: 65156194. [ Links ]
29. Nazzal H, Duggal MS. Regenerative endodontics: a true paradigm shift or a bandwagon about to be derailed? Eur Arch Paediatr Dent. 2017 Feb;18(1):3-15. PubMed PMID: 28092093. Pubmed Central PMCID: PMC5290056. [ Links ]
30. Bradaschia-Correa V, Casado-Gomez I, Moreira MM, Ferreira LB, Arana-Chavez VE. Immunolocalization of Smad-4 in developing molar roots of alendronate-treated rats. Archives of Oral Biology. 2013 Nov;58(11):1744-50. PubMed PMID: 23827715. [ Links ]
31. Sykes LM, Uys A, Bernitz H. The importance of record keeping in Forensic Odontology: a case discussion and general medico-legal guidelines for all practitioners. SADJ. 2016;71(5):224-7. [ Links ]
 Correspondence:
Correspondence:
Sulette Nel
Department of Oral Pathology and Oral Biology
School of Dentistry, Faculty of Health Sciences
University of Pretoria.
E-mail: Sulette.nel@up.ac.za














