Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.72 no.6 Johannesburg Jul. 2017
http://dx.doi.org/10.17159/2519-0105/2017/v72no6a8
CLINICAL REVIEW
Comparison of alveolar osteitis with post implant removal osteitis (Can a "dry socket" occur after implant removal?)
LM SykesI; D HerbstII; H DullabhIII; N FernandesIV
IBSc, BDS, MDent (Pros), Dip Res Ethics (Irensa), Dip For Odont. Department of Prosthodontics, University of Pretoria
IIBSc, BChD, MChD (Pros), FCD(SA). Department of Prosthodontics, University of Pretoria
IIIBChD, MSc (Dent), MDent (Pros). Department of Prosthodontics, University of Pretoria
IVBDS (Wits). Department of Prosthodontics, University of Pretoria
MOTIVATION
The introduction of dental implants spawned an exponential growth in the number of fixtures being placed to meet the increasing functional and aesthetic demands of patients. In response, manufacturers have flooded the market with new, cheaper systems, and many general practitioners have begun placing implants to support restorations. Enhanced life expectancy means that implants placed in younger people are expected to function effectively over many years. Studies have shown that a certain low percentage of implants will develop early or late complications,1 and that the risks are greater with increased usage.2 It is thus anticipated that practitioners will be faced with increasing numbers of implant-related complications that will require appropriate management, or even implant removal.1 Hence the majority of complications will be in older persons where healing may be compromised due to physiological ageing, systemic medication, or other age-related factors.3
The literature is replete with references of difficulties associated with implant placement, as well as reasons for implants requiring removal, but very little is mentioned on any problems following implant removal. Early removal is generally easy as the fixture will not yet have integrated with the surrounding bone. Late removal may be more difficult, especially if there are still areas of bony union, and could potentially result in trauma to the surrounding bone, similar to that seen after a difficult tooth extraction. It is thus postulated that a condition of post implant removal osteitis (it shall be referred to as PIRO in this paper) could develop, which may resemble post extraction alveolar osteitis (AO). If this is so it may be prudent to advise dentists to take extra precautions when removing implants in high risk patients or situations, and to avoid immediate replacement with new implants in conditions where there is a possibility of PIRO.
This paper provides a brief review of AO in terms of aetiology, pathogenesis, treatment, and prevention, as well as a brief overview of peri-implantitis (PI). It then explores reasons for implant removal and expands on the possibility that a similar condition to AO may be encountered post implant removal.
INTRODUCTION
Alveolar osteitis (AO)
This is a relatively common post-extraction complication resulting in inflammation of the extraction socket which is accompanied by intense throbbing pain within and around the extraction site.4 It begins within the first 24 hours after extraction, and increases in severity if left untreated.5 It is usually due to loss or disintegration of the blood clot in the base of the socket, with resulting accumulation of bacteria and food debris in the socket, and a distinctive malodour / halitosis.4,6 Reported frequencies vary from <1% to 19.14%, with an average range of about 1.7% following non-surgical extractions to 15% after surgical removal.5
Aetiology
This is multifactorial and many factors have been reported to predispose to an increased risk of development of AO. They include procedures involving flap reflection, excessive grinding and removal of bone; tooth splitting leading to tooth and bone fragments remaining in the socket;4 flap design (especially in third molar surgery); poor oral hygiene; pre-operative infection; traumatic extractions causing compression of the bone lining the socket; thrombosis of underlying vessels;4 smoking which retards healing; increased age; systemic disorders; single extraction sites; extraction of impacted third mandibular molars as well as other mandibular teeth with thick cortical bone and / or poor blood networks;4 use of large amounts of local anaesthetics; intra-ligamentous injections; antibiotic use prior to surgery; difficult surgery, increased surgical time, or poor surgical techniques; the use of certain post-operative analgesics (specifically ibuprofen); previous osteomyelitis; extraction in irradiated bone; and post extraction irrigation with saline or water which interferes with blood clot formation,5 (recognising that accepted literature advocates socket irrigation to remove bone and tooth debris that could impede healing.7) In females; the use of oral contraceptives containing oestrogen which affects coagulation; 4,5,8 and extractions in the middle stages of the menstrual cycle are additional possible predisposing factors.
Pathogenesis
Fibrinolysis is a physiologic process whereby fibrin is laid down and then may be removed from the body by enzymatic digestion as part of ongoing healing and repair. Plasminogen is incorporated in the fibrin network as it forms.4 Later, lysis of the blood clot occurs due to the action of tissue kinases liberated during inflammation by direct or indirect activation with conversion of plasminogen to plasmin in the blood. Plasmin acts to dissolve the clot. Direct activators such as tissue and endothelial plasminogen activators are normally present. However, indirect activators such as streptokinase and staphylokinase, are produced by bacteria and bind to the plasminogen causing its activation to plasmin and speeding up the clot dissolution. This confirms the theory for bacterial involvement in AO development.4 The pain characteristic of AO is due to the presence of kinins within the socket.9 Many organisms have been cultured from infected sites including Capnocytophaga, Fusobacteria, Streptococci, Treponema,5 Actinomyces,10 and other anaerobes.11 Many of these bacteria secrete pyrogens which are indirect activators of fibrinolysis.4 Infection results in the host producing high levels of serum-C reactive protein which increases the potential for dissemination of infection, as well as disturbing alveolar repair processes.5
Treatment
Treatment consists of irrigation, surgical curettage and antibacterial or analgesic dressing, with or without adjunctive antibiotics. Alvogyl (benzocaine, balsam of Peru and eugenol) is a commonly used dressing due to its immediate pain relief, low cost, ease of use and favourable outcomes.4 Various other medicaments have also been tested, such as zinc oxide eugenol (ZOE) on a gauze strip, thermosetting gels (2.5% prilocaine and 2.5% lidocaine), SaliCept, and pastille GECB (3% gualacol, 3% eugenol, 1.6% chlorobutanol).5 Plasma rich growth factors (PRGF) have also been used to speed up healing, but relief of pain is more effective with conventional ZOE gauze.4,5 Recent studies show improved healing in those treated with curettage, irrigation and continuous mode diode laser irradiation.5 Antimicrobial photodynamic therapy (aPTDT) with HELBO Blue and TheraLite lasers may help decontaminate extraction sockets, and could be used for prevention and/or treatment of AO.4 If necessary, antibiotics may be prescribed, most commonly amoxicillin.5
Prevention
The use of prophylactic antibiotics is controversial, given the hazards of unnecessary and over-prescription. It should be restricted to those with a history of AO or immunocompromised patients.5 While some authors advocate prophylactic use of azithromycin,5 penicillin, clindamycin, erythromycin and metronidazole,12 other investigators found no difference between patients given prophylactic amoxicillin to those without antibiotic cover.13 The placement of sutures and haemostatic agents prolongs operative time, a predisposing factor. The use of chlorhexidene (0.12 -0.2% concentrations) as a pre-operative irrigant and post-operative mouthrinse has been shown to significantly reduce the incidence of AO.5,6,14 More recent studies have investigated various topical gels such as "gelatamp" (colloidal silver impregnated sponges), parahydroxybenzoic acid, tranexamic acid, polymer polylactic acid, and chlorhexidene gel (0.2%) to help prevent AO. Results were inconclusive for most except chlorhexidine gel, which was found to remain the best medicament for prevention of AO.4-6 Ultimately, one of the most critical preventive measures is the maintenance of a sterile surgical environment.4
OVERVIEW OF PERI-IMPLANT MUCOSITIS AND PERI-IMPLANTIS
Aetiology
Peri-implant mucositis (PIm) is a reversible inflammation of the soft tissues surrounding a functioning osseointegrated implant with no loss of the supporting bone. Peri-implantitis (PI) is an inflammatory process affecting the tissues around a functioning osseointegrated implant resulting in the loss of supporting bone.15 Clinically PIm presents with bleeding on probing with/without suppuration, and probing depths of 4-5mm. PI has deeper probing depths and progressive supporting bone loss beyond biological bone remodelling. Pain is seldom a feature of either disease, and progression is usually slow.16,17 Patient risk factors include poor oral hygiene; design of the overlying prosthesis (which may hamper good oral hygiene practices); lack of keratinised mucosal attachment which predisposes the soft tissue to mechanical damage and plaque accumulation; history of previous periodontitis;18 failure to follow a regular maintenance programme; genetic traits influencing host inflammatory responses;19 diabetes; smoking; and alcohol consumption.17,20
Surgical and prosthodontic implant risk factors include implant site (both anterior mandible and anterior maxilla have been associated with increased risks of PI associated bone loss);21,22 implants placed too deeply or too close to each other, with overcompression of adjacent bone; insufficient irrigation during placement;3,23 immediate placement and immediate loading;24 microgaps at the bone level;25 residual cement in peri-implant tissues; over-contoured or poorly designed prostheses which prevent adequate oral hygiene;21 occlusal overload;26 full rehabilitation as opposed to single crown replacement; foreign body reactions to certain metallic components;27 and restorations which are carried out by general practitioners as opposed to specialists.28 Neither different flap designs24 nor implant surfaces17 had significant effects on the development of PI, whilst platform switching is believed to reduce its incidence.26
Pathogenesis
Marginal bone loss is mainly due to bacterial infection and is mediated by biofilms similar to that in natural dentition. The host responds to this biofilm on the implant surface by a series of inflammatory reactions, initially confined to the soft tissues, but later progressing deeper. Deep pockets around the implant create favourable anaerobic environments for periodontal pathogens, but these micro-organisms may not be solely responsible for the initial bone resorption. Often there are underlying implant, patient or clinician related factors that initiate the inflammatory process, which is later exacerbated by bacterial infection.19,29 Following the initial inflammatory process, certain immune cells (macrophages, neutrophils, lymphocytes and plasma cells) provoke tissue damage. Pro-inflammatory cytokines in the form of interleukins and tumour necrosis factor are upregulated, and enhance the inflammatory response leading to tissue damage. Once the soft tissue peri-mucosal seal has been compromised, bone destruction usually follows.3,15,20,30
REASONS FOR IMPLANT REMOVAL
Dental implants may develop a variety of biological or biomechanical complications. These include inflammation and infection of the surrounding soft tissues, severe bone involvement and loss, and structural or mechanical failures. There is no clear consensus on how to treat a failing implant. If it is due to bacterial-host responses, conservative debridement with antiseptics and adjunct antibacterial drugs is a first line approach. More severe bone loss requires more invasive surgical approaches combining implant surface decontamination with guided bone regeneration procedures.31 Implant removal may be needed in cases of persistent infection; significant bone loss; pain, fractures, incorrectly positioned implants that cannot be restored, implant mobility, lack of bone coverage, advanced gingival recession with implant thread exposure, and fractured screws that cannot be retrieved.32 Fractured cross headed screws are almost impossible to remove and may result in the need to remove the entire implant, despite it being fully integrated.33
Reports have shown that just under 1% of implants placed could fracture, especially in partially dentate cases, and in posterior regions where occlusal stresses are the highest.2 Later studies34 showed that these fractures were mostly due to metal fatigue as opposed to material corrosion. Depending on the level of the fracture, most of these implants are unrestorable and need removal. A fractured implant, or one with a fractured screw, may still be fully integrated, thus removal procedures could be potentially damaging to surrounding bone, increasing the risk of PIRO.
Various instruments may be used to remove a failing implant, and selection should be based on those which will produce the least tissue damage. Unfortunately, if there are areas where the implant remains tightly integrated, the surrounding bone is often compromised in the process.32 This may lead to complications, one of which occurred on a patient treated at the University of Pretoria Oral and Dental Hospital. This patient developed a localized osteitis following implant removal. The site ("socket") was treated conservatively following the protocol used for AO, and healing was uneventful.
COMPARISON OF AO AND PIRO
Implants are very different to teeth in that the surrounding soft and hard tissues are both devoid of an independent blood supply. This results in reduced immunological defences against injury. Furthermore there is a weaker mucosal seal as there are fewer attachment fibres around implants which run more vertically and attach to the alveolar crest, as opposed to the larger amounts of horizontally oriented Sharpey's fibres which attach to a tooth's cementum.35 There is merely an abutting of soft scar-like connective tissue against the implant surface. The resulting weaker peri-mucosal seal around implants allows for easier bacterial penetration. Despite this, these conditions are remarkably similar, with few notable differences (Table 1).
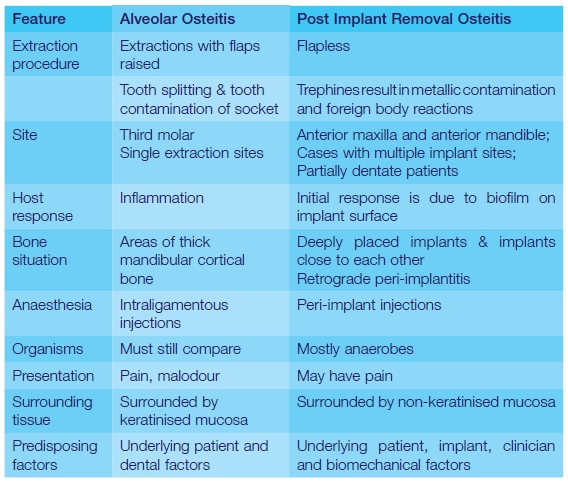
CONCLUSIONS
Despite differences, many similarities support the notion that PIRO is clinically similar to AO and thus preventive and treatment strategies should also be similar. The final message for clinicians is that they should be alert to patients and situations where there is an increased risk of developing PIRO. These include implant removal in older patients, females, partially dentate cases, fractured components, deeply placed fixtures, immunocompromised hosts, and those where removal has resulted in traumatic bone injury. It is advised that immediate implant replacement be not carried out in these cases as subsequent osseointegration may be complicated by the development of PIRO. A replacement implant should only be considered after soft tissue closure, with complete resolution and healing of the site. This can be verified by a periapical radiograph, and will also reveal whether further bone grafting is needed before a new implant is placed. Proceed with caution as this patient would already be classed as high risk for complications.
ACRONYMS
AO: alveolar osteitis
PI: peri-implantitis
PIm: Peri-implant mucositis
PIRO: photobiomodulating
References
1. Greenstein G, Cavallaro, J: Failed dental implants: diagnosis, removal and survival of reimplantations., J Am Dent Assoc 2014, 145:835-42 [ Links ]
2. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JYK: Clinical complications with implants and implant prostheses, J Prosthet Dent 2003, 90:121-32 [ Links ]
3. Bragger U, Heitz-Mayfield LJA: Biological and hardware complications in implant dentistry, ITI treatment guide 2015, 8:1-198 [ Links ]
4. Sheikh M, Kiyani, A., Mehdi, A., Musharaf, Q: Pathogenesis and management of dry socket (Alveolar osteitis). Pakistan Oral and Dental Journal 2010, 30:323-5 [ Links ]
5. Tarakji B, Saleh, LA., Umair, A., et al: Systemic review of dry socket: Aetiology, treatment and prevention., J Clin and Diag Research 2015, 9:10-3 [ Links ]
6. Daly B, Sharif, MO., Newton, T., et al: Local interventions for the management of alveolar osteitis (dry socket). Cochrane Database of Systematic Reviews 2012 2012, Art No: CD006968 [ Links ]
7. Blum I: Contemporary views on dry socket: a clinical appraisal of standardization, aetiopathogenesis and management: a clinical review., Int J Oral and Maxillofac Surg 2002, 31:309-17 [ Links ]
8. Garcia A, Grana, PM., Samporedro, FG., Diago, MP et al: Does oral contraceptive use affect the incidence of complications after extraction of a third molar., Br Dent J 2003, 194:453-455 [ Links ]
9. Birn H: Kinins and pain in dry socket., Int J Oral Surg 1972, 1:32-42 [ Links ]
10. Razanis J, Schofield, IDF., Warren, BA.: Is dry socket preventable?, J Can Dent Assoc 1997, 43:233-6 [ Links ]
11. Nitzan D: On the genesis of dry socket., J Oral and Maxillofac Surg 1983, 41:706-10 [ Links ]
12. Siddiqi A, Morkel, JA., Zafar, S.: Antibiotic prophylaxis in third molar surgery: a randomized, double blind placebo-controlled clinical trial using split mouth technique., Int J Oral Surg 2010, 39:107-14 [ Links ]
13. Bezerra T, Studart-Soares, EC., Scaparo, HC., et al: Prophylaxis versus placebo treatment for infective and inflammatory complications of surgical third molar removal: a split-mouth, double blind, controlled, clinical trial with amoxicillin (500mg). J Oral Maxillofac Surg 2011, 69:e333-339 [ Links ]
14. Caso A, Hung, LK., Beirne, OR.: Prevention of alveolar osteitis with chlorhexidine: a meta analytical view., 2005, 99:155-9 [ Links ]
15. Heitz-Mayfield LJ: Diagnosis and management of peri-implant diseases, Australian Dental Journal 2008, 53 Suppl 1:S43-48 [ Links ]
16. Mombelli A, Muller N, Cionca N: The epidemiology of peri-implantitis, Clinical Oral Implants Research 2012, 23 Suppl 6:67-76 [ Links ]
17. Nguyen-Hieu T, Borghetti A, Aboudharam G: Peri-implantitis: from diagnosis to therapeutics, Journal of Investigative and Clinical Dentistry 2012, 3:79-94 [ Links ]
18. Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Bragger U, Hammerle CHF, Lang NP: Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI dental implant system, Clinical Oral Implants Research 2003, 14:329-39 [ Links ]
19. Klinge B: Peri-implant marginal bone loss: an academic controversy or a clinical challenge?, Eur J Oral Implantol 2012, 13-9 [ Links ]
20. Heitz-Mayfield LJ: Peri-implant diseases: diagnosis and risk indicators, J Clin Periodontol 2008, 35:292-304 [ Links ]
21. Fransson C, Wennstrom J, Tomasi C, Berglundh T: Extent of peri-implantitis-associated bone loss, J Clin Periodontol 2009, 36:357-63 [ Links ]
22. Koldsand OC, Scheie AA, Aass AM: The association between selected risk indicators and severity of peri-implantitis using mixed model analyses, J Clin Periodontol 2011, 38:285-92 [ Links ]
23. Trisi P, Berardini M, Falco A, Podaliri Vulpiani M, Perfetti G: Insufficient irrigation induces peri-implant bone resorption: an in vivo histologic analysis in sheep, Clinical Oral Implants Research 2014, 25:696-701 [ Links ]
24. Quirynen M, Van Assche N, Botticelli D, Berglundh T: How does the timing of implant placement to extraction affect outcome?, Int J Oral Maxillofac Implants 2007, 22(Suppl):203-23 [ Links ]
25. Hermann JS, Cochran DL, Nummikoski PV, Buser D: Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the canine mandible, J Periodontol 1997, 68:1117-30 [ Links ]
26. Qian J, Wennerberg A, Albrektsson T: Reasons for marginal bone loss around oral implants, Clinical Implant Dentistry and Related Research 2012, 14:792-807 [ Links ]
27. Albrektsson T, Canullo L, Cochran D, De Bruyn H: "Peri-Implantitis": a complication of a foreign body or a man-made "disease". Facts and Fiction. Clinical Implant Dentistry and Related Research 2016, 18:840-9 [ Links ]
28. Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T: Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. Journal of Dental Research 2016, 95:43-9 [ Links ]
29. Mombelli A, Decaillet F: The characteristics of biofilms in peri-implant disease, J Clin Periodontol 2011, 38 Suppl 11:203-13 [ Links ]
30. Zhuang LF, Watt RM, Mattheos N, Si MS, Lai HC, Lang NP: Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues, Clinical Oral Implants Research 2016, 27:13-21 [ Links ]
31. Ramanauskaite A, Daugela P, Juodzbalys G: Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal, Quintessence Int 2016, 47:379-93 [ Links ]
32. Bragger U, Karoussis IK, Persson R, Pjetursson B, Salvi GE, Lang NP: Technical and biological complications/failures with single crowns and fixed partial dentures on implants: a 10-year prospective cohort study, Clinical Oral Implants Research 2005, 16:326-34 [ Links ]
33. Sailer I, Muhlemann S, Zwahlen M: Cemented and screw retained implant reconstructions: a systematic review of the survival and complication rates, Clinical Oral Implants Research 2012, 23:163-201 [ Links ]
34. Shemtov-Yona K, Rittel D: Identification of failure mechanisms in retrieved fractured dental implants, Engin Failure Analy 2014, 38:58-65 [ Links ]
35. Wang Y, Zhang Y, Miron RJ: Health, maintenance, and recovery of soft tissues around implants, Clinical Implant Dentistry and Related Research 2016, 18:618-34 [ Links ]
 Correspondence:
Correspondence:
Leanne M Sykes
Associate Professor and Head Clinical Unit, Faculty of Health Sciences
University of Pretoria. Oral and Dental Hospital, University of Pretoria
Private Bag X20, Hatfield 0028, South Africa
Tel: +27 (0)12 319 2683
E-mail: Leanne.sykes@up.ac.za














