Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.72 n.3 Johannesburg Apr. 2017
CLINICAL REVIEW
Management of necrotic pulp of immature permanent incisor tooth: A regenerative endodontic treatment protocol: case report
DS MoodleyI; C. PeckII; T MoodleyIII; N PatelIV
IPhD, MSc Dent, PDD Aesth, BDS. 'Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
IIBChD''Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
IIIBChD. 'Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
IVMChD'. Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
ABSTRACT
It is possible that a paradigm shift may be in the offing in the approach to treatment of immature teeth with necrotic pulp, away from traditional apexification procedures and to a biologically-based endodontic protocol intended to produce regeneration, based on the deliberate introduction of bleeding into the canal space to provide a scaffold and allow the ingress of stem cells.
METHODS: A patient presented with a maxillary right central incisor tooth with an open apex and periapical radiolucency. The tooth was irrigated with sodium hypochlorite and then dressed with tri-antibiotic paste consisting of ciprofloxacin, metronidazole and amoxicillin. At a subsequent visit a blood clot was produced in the canal by irritating periapical tissues and the canal then sealed with mineral trioxide aggregate and glass ionomer cement.
RESULTS: The patient was pain free, the draining sinus was resolved in two weeks, root maturation continued and apical closure occurred after two months. The tooth became responsive to cold pulp vitality testing.
CONCLUSIONS: Continued root growth invoked by regenerative endodontics may reduce the risks of fracture and premature tooth loss otherwise associated with traditional CaOH2 apexification procedures. Randomised, prospective clinical trials and long term studies are required before the technique becomes standard practice.
Keywords: Endodontics, Regeneration, Revascularization, Revitalization, Stem Cells, Apexifixation, Apexogenesis.
INTRODUCTION
Endodontic management of the immature permanent tooth with an open apex is a challenge to clinicians. Their concerns when treating teeth with "blunderbuss" canals are primarily related to adequacy of disinfection of the canal space and obturation difficulties, especially in controlling working length.1 Long-term studies of apexification procedures to close the root-end in the treatment of a non-vital pulp of an immature tooth with calcium hydroxide have proven the successful retention of many of these infected immature permanent teeth. When apexification is undertaken, however, it is accepted that there will be no more development of the root in terms of apical maturation and thickening of its dentine walls.2 Hence these teeth have been found to be prone to cervical fracture and subsequent loss, due to their thin dentinal walls and perceived problems associated with long-term placement of calcium hydroxide.3-5 Those unfavourable outcomes have led to the development of endodontic procedures such as pulp revitalization as an alternative to the use of Ca(OH)2 for the treatment of infected pulps in immature permanent teeth. Indeed, if evidence-based research substantiates the potential, there could be a paradigm shift in the treatment of immature teeth with necrotic pulp from traditional apexification procedures to a biologically-based regenerative endodontic protocol.6
Recently there has been an increased interest in the possibility of regenerating pulp tissue into a previously infected immature tooth. Regenerative endodontics is considered as one of the most exciting prospective developments in dentistry today.7 Novel advances in biotechnology have made it possible to provide treatment modalities that protect the vital pulp, allow manipulation of reactionary and reparative dentinogenesis, and, more recently, permit revascularization of an infected root canal space.8 These approaches are referred to as 'regenerative' procedures, and in endodontics are biologically- based and designed to replace damaged tissues, including dentin and root structures, as well as cells of the pulp-dentin complex. 'Revascularization' was adapted as a term by Iwaya et al.9to describe the clinical healing of periapical abscesses and the continuation of root formation in immature teeth with non-vital pulps.2 The term simply means the re-establishment of vascularity in the pulp space. With that rich blood supply comes the possibility of actual tissue regeneration, of cementum, periodontal ligament, bone, and dentin and, most relevantly, of the pulp, restoring functional properties of the tooth. Continued root development may thereby be fostered in immature teeth, and apical periodontitis resolved or prevented.10 Thus, the alternative term of "revitalization" has been proposed.2 Permanent teeth with immature apices have a rich cellular and vascular supply and so dental pulp stem cells (DPSC) and stem cells of apical papilla (SCAP) may survive disinfection, as has been shown when immature teeth with pulpal necrosis still undergo apexogenesis.11,12 The maintenance of pulp vitality is particularly critical in young patients with incomplete root formation13 and hence the gain in interest in this endodontic treatment protocol for the management of the immature permanent tooth with an open apex. Regenerative endodontic procedures can allow for resolution of apical periodontitis and associated draining sinus tracts and enable continued root maturation.6 The rationale of revascularization is that if a sterile tissue matrix is provided in which new cells can grow, pulp vitality can be re-established.14 A newly created blood clot formed as a result of deliberately induced bleeding into the canal space provides that matrix. Growth factors like platelet derived growth factor, vascular endothelial growth factor and tissue growth factor are present in the clot and can stimulate the differentiation of undifferentiated cell types. The scaffold provides a physico-chemical and biological three-dimensional micro environment for cell growth and differentiation, promoting cell adhesion and migration.15 Stem cells and progenitor cells from the pulp (DPSC) and/or periodontium (SCAP) contribute to continued root development.6
CASE REPORT
Patient History
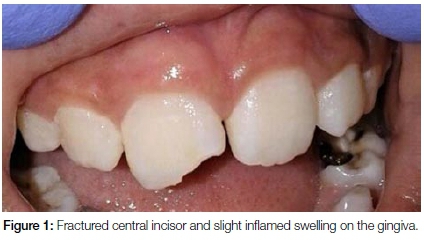
A healthy 10-year-old boy was referred to the Tygerberg Oral Health Centre for evaluation and treatment of a dental abscess. On clinical examination, swelling of the upper lip was noted. Intra-oral examination revealed a fractured crown on upper right central incisor, tooth number 11 (Figure 1) with pulpal exposure. A gingival swelling was present in the apical region and slight mobility of the tooth was observed. Radiographically, a periapical radiolucency was associated with the 11, which was also seen to have an open apex (Figure 2).

According to the American Association of Endodontists (AAE) recommendations 16 for the endodontic management of the permanent immature tooth with an open apex, the patient should fulfil the following criteria:
• Tooth with necrotic pulp and an immature apex.
• Pulp space not needed for post/core, final restoration.
• Compliant patient/parent.
• Patients not allergic to medicaments and antibiotics necessary to complete procedure (ASA 1 or 2).
The presenting patient fulfilled the AAE requirements for the management of the traumatised 11.
Informed consent
Informed consent from the parent was obtained for the following:
• Two or more appointments may be required for this treatment.
• Use of antimicrobials.
• Possible adverse effects: staining of crown/root and lack of response to treatment, pain/infection.
• Alternatives treatment options such as apexification, no treatment or extraction were presented.
First appointment
Local anaesthesia, dental dam isolation and access into the pulp chamber were obtained. The canal space was cleaned using 20ml 1.5% NaOCl applied through a side-vented needle that minimized the possibility of extrusion of irrigants into the periapical area. The irrigating needle was positioned about 1 mm from root end, to reduce cy-totoxicity to cells in the apical tissues. The canal was then irrigated with saline and dried with paper points. Triple antibiotic paste, combining ciprofloxacin, metronidazole and minocycline with saline to a final concentration of 1.0 mg/ml, as described by Sato et al.17was placed into the canal system using a syringe. Since there is a possibility of staining with triple antibiotic, the paste was kept below the CEJ. The remaining space was restored with an initial layer of calcium hydroxide and sealed with 3-4mm of glass-ionomer temporary restoration material (Vitremer, 3M ESPE). The patient was dismissed for two weeks.
Second appointment
The patient was assessed for signs/symptoms of persistent infection. The swelling on the gingiva was completely resolved and patient was free of any discomfort. Anaesthesia using 3% mepivacaine without vasoconstrictor was obtained and the tooth was isolated with a dental dam. The canal space was gently irrigated with 20ml of 17% EDTA and dried with paper points. Bleeding was created into canal system by over-instrumenting and rotating a pre-curved K-file at 2mm past the apical foramen with the goal of having the entire canal filled with blood to the level of the cemento-enamel junction. Bleeding was stopped at a coronal level that allowed for about a 3-4mm space. Mineral trioxide aggregate (MTA; Dentsply Tulsa Dental, Tulsa, USA) was carefully placed over the blood clot followed by glass ionomer restorative material (Vitremer, 3M ESPE).
Two-month follow-up appointment
Clinical and radiographic examination: No pain, soft tissue swelling or sinus tract was present. Resolution of the apical radiolucency with slightly increased width of root walls and closure of the apex was noted radiographically (Figure 3). A positive response was observed when the tooth was subjected to pulp vitality testing.

Five-month follow-up appointment
Radiographically (Figure 4) tooth number 11 reveals closure of apex five months' post treatment. Clinically the tooth was vital, responding to cold stimulus.

DISCUSSION
Conventionally, it has been thought that successful regeneration cannot be an expected outcome after a tooth has become infected. However, there is a growing body of evidence to suggest that regenerative endodontics may in fact be possible in teeth with pulpal necrosis, apical pathosis, and immature apexes.18
The American Association of Endodontists (AAE) recognizes the potential in regenerative endodontics and hence has made the protocol a strategic priority.16 The AAE Regenerative Endodontics Committee encourages en-dodontists to submit their cases to: http://www.aae.org/ members/revascularizationsurvey.htm to create a larger data base for research. The degree of success of regenerative endodontic procedures, as laid out by the AAE, is largely measured by the extent to which it is possible to attain primary, secondary, and tertiary goals.16
Primary goal: The elimination of symptoms and evidence of bony healing.
Secondary goal: Increased root wall thickness and/or increased root length (desirable, but perhaps not essential).
Tertiary goal: Positive response to vitality testing (which could indicate a more organized and vital pulp tissue).
Most studies on regenerative procedures have been conducted on teeth with open apices. The size of the apical foramen may not be the decisive factor for successful revascularization and ingrowth of new tissue after transplantation. The minimum width of the apical foramen has not been determined, but it has been shown that a size smaller than 1mm does not prevent revascularization and ingrowth of vital tissue.19
With regards to the type of irrigant to be used in regenerative endodontic procedures, a balance needs to be made between elimination of bacteria and at the same time maintaining the vitality of the stem cells. Because of the possibility of extruding NaOCl through the open apex, risking cytotoxicity to the apical stem cells,20 the AAE recommends the use of as low a concentration of the irrig-ant as possible.16 However, a subsequent rinse with 17% EDTA could minimise any toxic effects, and allow recovery of cell viability.21 EDTA has another positive effect in that it stimulates the release of growth factors on the dentine surface.7 Mechanical cleaning may further weaken the thin root canal walls22 and remove vital tissue remnants that might still be present in the apical parts of the canal, and hence should be avoided during this procedure.
Intracanal medication with the use of a combination of three antibiotics, ciprofloxacin, metronidazole and minocycline, called triple antibiotic paste (TAP), efficiently eliminates the bacteria commonly found in infected root canal dentine17,23,24 and has been widely used for revitalization therapies with good success.13 However, since minocycline may cause discolorations, this antibiotic was more recently replaced by cefaclor in a modified mTAP.25 Other practitioners26 have omitted it completely and have used a double antibiotic paste, DAP, while Cachreli et al. used CaOH2 as a medicament.27 There are concerns of antibiotic resistance, risk of patient sensitization and the fact that antibiotic pastes may negatively influence growth factor release from dentine by EDTA, a process which is actually increased after the use of calcium hydroxide.28 However, regenerative endodontic treatment with triple antibiotic paste produced significantly greater increases in root length and wall thickness than either the mineral trioxide aggregate (MTA) apexification or non-surgical root canal treatment control groups.29 In a clinical study involving teeth in 14 patients, 93% of the teeth showed resolution of peri-radicular radiolucencies, and thickening of lateral dentinal walls was seen in 57%, with increased root length in 71% of the cases.30 None of the patients presented with pain, reinfection or radiographic enlargement of preexisting periapical lesions.30
In the case under report, a blood clot was created in the canal after disinfection to act as a matrix for the growth of new tissue into the pulp space. Dental pulp has a high regenerative capacity because of the presence of progenitor cells and inductive regeneration signals from different origins.31 Apical closure was seen after five months. The nature of the tissue formed within the canal space in this and similar cases is not clear and is currently controversial. Some histologic studies suggest that the calcified material deposited on the canal wall is bone/cementum rather than dentine, hence the absence of pulp tissue with or without an odontoblast layer.3 Continued root development is seen, mostly by cellular/acellular cementum and histologically, the most apical portion of the canal shows newly formed mineralized tissue.32,33 Immuno-histochemical analysis has shown that the tissue formed inside the root canal is positive to bone sialoprotein (BSP) but negative to dentine sialo-protein (DSP).32 Histologic evidence of hard-tissue deposition on the root canal walls (43.9%), apical closure (54.9%), and formation of vital tissue in root canal space (29.3%) was demonstrated by Thibodeau et al.34in a dog model.
It is relevant to note that failures have been experienced with this treatment protocol, have been reported and are mainly associated with reinfection of the root canal system, root resorption possibly as a result of inadequate removal of biofilm, the presence of bacteria in dentinal tubules or defective restoration.4
CONCLUSION
This case illustrates success in the short term, mainly in the closure of the apex and a thickening of the dentinal walls. Although promising results have been reported, it is clear that randomized clinical trials are required to assess the long-term clinical outcomes of this innovative treatment protocol.13
ACRONYMS
AAE: American Association of Endodontists
DPSC: dental pulp stem cells
EDTA: Ethyl diamino tetra acetic acid
SCAP: stem cells of apical papilla
TAP: triple antibiotic paste
References
1. Trope M. Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am. 2010;54:313-24. [ Links ]
2. Huang GTJ. Apexification: The beginning of its end. Int Endod J. 2009;42(10):855-66. [ Links ]
3. Harlamb S. Management of incompletely developed teeth requiring root canal treatment. Aust Dent J. 2016;61(S1):95-106. [ Links ]
4. Conde MCM, Chisini LA, Sarkis-Onofre R, Schuch HS, Nör JE, Demarco FF. A scoping review of root canal revascularization: relevant aspects for clinical success and tissue formation. Int Endod J. 3 Dec 2016 doi:10.1111 /iej.12711. [ Links ]
5. Andreasen JO, Bakland LK. Pulp regeneration after non-infected and infected necrosis, what type of tissue do we want? A review. Dental Traumatol. 2012 ;28 : 13-8. [ Links ]
6. Thomson A, Kahler B. Regenerative endodontics - Biologically- based treatment for immature permanent teeth: A case report and review of the literature. Aust Dent J. 2010;55(4):446-52. [ Links ]
7. Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller K-A, Hoffer PC, et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int En- dod J. 2016;49(6):581-90. [ Links ]
8. Simon SRJ, Tomson PL, Berdal A. Regenerative endodontics: regeneration or repair? J Endod. 2014 Apr;40(4Suppl):S70-5. doi:10.1061/j.joen 2014.01.24. [ Links ]
9. Iwaya S, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17(4):185-7. [ Links ]
10. Neha K, Kansal R, Garg P, Joshi R, Garg D, Grover H-S. Management of immature teeth by dentin-pulp regeneration: a recent approach. Med Oral Patol Oral Cir Bucal Nov. 2011;116(7):997-1004. [ Links ]
11. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J Endod. 2004;30(4):196-200. [ Links ]
12. Chueh LH, Huang GTJ. Immature teeth with periradicular peri-odontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32(12):1205-13. [ Links ]
13. Galler KM. Clinical procedures for revitalization: current knowledge and considerations. Int Endod J. 2016;49(10):926-36. [ Links ]
14. Sridharan S, Neelakantan P. Revascularization in endodontics. Int J Clin Dent. 2014;7(2):139-45. [ Links ]
15. Deepak B, Nandini D, Naik S. Tissue Engineering : Is it the future of endodontics? People's J Sci Res. 2011;4(1):76-82. [ Links ]
16. American Association of Endodontics. AAE Clinical Considerations for a Regenerative Procedure [Internet]. American Association of Endodontics. 2014. Available from: www.aae.org/Dental_Professionals/Considerations_for_Regenerative_Procedures.aspx. _and_research/research/currentregenera-tiveendodonticconsiderations.pdf. Accessed December 2015 [ Links ]
17. Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29(2):118-24. [ Links ]
18. Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endod. 2012;38(6):864-8. [ Links ]
19. Laureys WGM, Cuvelier CA, Dermaut LR, De Pauw GAM. The critical apical diameter to obtain regeneration of the pulp tissue after tooth transplantation, replantation, or regenerative endodontic treatment. J Endod. 2013; 39(6):759-63. [ Links ]
20. Siqueira JF, Rögas IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after in strumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26(6):331-4. [ Links ]
21. Martin DE, De Almeida JFA, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40(1):51-5. [ Links ]
22. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol [Internet]. 1992;8(2):45-55. [ Links ]
23. Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29(2):125-30. [ Links ]
24. Takushige T, Cruz E V., Asgor Moral A, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37(2):132-8. [ Links ]
25. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29(1):47-50. [ Links ]
26. Iwaya S, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol Traumatol C Munksgaard Welf Pension Hosp. 2001;17(17):185-7. [ Links ]
27. Cehreli ZC, Isbitiren B, Sara S, Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a Case Series. Journal of Endodontics. 2011; 37(9): 1327-30. [ Links ]
28. Galler KM, Buchalla W, Hiller KA, Federlin M, Eidt A, Schiefersteiner M, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41(3):363-8. [ Links ]
29. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35(10):1343-9. [ Links ]
30. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34(8):919-25. [ Links ]
31. Chmilewsky F, Jeanneau C, Dejou J, About I. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J Endod. 2014;40(4S):19-25. [ Links ]
32. Shimizu E, Ricucci D, Albert J, Alobaid AS, Gibbs JL, Huang GT-J, et al. Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. Vol. 39, Journal of Endodontics 2013;39(8): 1078-83. [ Links ]
33. Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. Journal of Endodontics. 2014; 40: 133-9. [ Links ]
34. Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33(6):680-9. [ Links ]
 Correspondence:
Correspondence:
Desigar S Moodley
Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape
Private Bag X1, Tygerberg, 7505
Cape Town, South Africa
Tel: +27 21 9373090
E-mail: dmoodley@uwc.ac.za














