Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.72 no.3 Johannesburg abr. 2017
CLINICAL REVIEW
Use of antibacterial nanoparticles in Endodontics
A I O IbrahimI; D S MoodleyII; L PetrikIII; N PatelIV
IMSc. Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
II.PhD, Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
IIIPhD. Department of Chemistry, Faculty of Natural Sciences, University of the Western Cape, Cape Town, South Africa
IVMChD. Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, Cape Town, South Africa
SUMMARY
Several root canal irrigants and medicaments are available to combat endodontic pathogens. However, evidence of complete elimination of these pathogens by the use of these solutions is not recorded in the literature. The possible development of resistant bacterial species is one of the problems related to the efficacy of the currently available irrigants and medicaments. In addition, the complex anatomy of the root canal system allows endodontic pathogens to be hidden in areas inaccessible to the action of the irrigating preparations. This is further enhanced by the protective layer that is formed by the remnants of pulp tissue, dentin powder and dead cells which inhibit the antibacterial activity of the root canal irrigants and medicaments. Antimicrobial nanoparticles show promising effect against resistant pathogens in pharmaceutical science as a result of their unique physio-chemical properties. Unlike traditionally used antimicrobial agents, these nanoparti-cles destroy bacterial cells through multiple mechanisms. The concept of using nanoparticles in endodontics as a new treatment modality was developed recently and their antibacterial efficacy against endodontic pathogens was evaluated by several researchers in many in vitro studies. This article reviews some of the currently available literature on laboratory studies that evaluated the efficacy of nanoparticles against endodontic pathogens.
Keywords: Endodontics, Antibacterial; chitosan; functionalized; nanoparticles; silver, magnesium, zinc.
INTRODUCTION
The world "nano" originated from a Greek word which means "dwarf".1 The philosophy of nanotechnology was first illustrated in 1960 by Richard P. Feynman, a Nobel Prize winner, in his lecture "There's Plenty of Room at the Bottom".2 Since then, the concept of nanotechnology has been applied in numerous scientific fields such as physics, engineering as well as in the medical field. Nanotechnology is defined as a science that deals with the development of new materials with new properties and functions through controlling and restructuring of the materials on a nanometer scale of "less than 100 nm" and hence the name nanomaterials.3 The term is applied, according to the European commission, to "any natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimension is in the size range 1 nm - 100nm".4
Nanomaterials exist in different forms and shapes. They are categorized according to their dimensions into: zero dimension such as nanoparticles, one dimension such as nanorods, two dimensions such as thin films and three dimensions such as nanocones.5 They show increased chemical reactivity compared with their bulk form.
The term nanodentistry is defined as "the science and technology of diagnosis, treating and preventing oral diseases, relieving pain, preserving and improving dental health using nanostructured material".6 Nanodentistry is applied in different areas, for example: manufacturing of dental materials; prevention of oral diseases such as dental caries and periodontal disease; as therapeutic agents for the treatment of dentine hypersensitivity, oral cancer and endodontic diseases; in the technology of tissue engineering; and as a diagnostic aid to identify certain diseases such as oral cancer.7 Currently the application of nanomaterial in endodontics is limited to a few studies that evaluated the antimicrobial properties of some nanomaterials in different forms against endodontic pathogens.
Endodontic diseases as a microbial infection
Microbial elements are the most common cause of pulpal and periapical pathosis.8 All endodontic infections are polymicrobial in nature9 with differences between the types of micro-organisms isolated from primary and secondary root canal infections.10 Microbiological studies have revealed more than 400 microbial species from endodontic samples.11
Endodontic infection is eventually established as a bio-film.12 This form of microbial colonization inside the root canal system was initially discovered by Ramachandran Nair.13 Microbial biofilm is a surface-attached microbial community defined by Mohammadi et al as "a sessile multicellular microbial community characterized by cells firmly attached to a surface and enmeshed in a self-produced matrix of extracellular polymeric substance".14 Endodontic biofilm is composed of 10-15% bacterial cells embedded in 85-90% of that extracellular substance.15
Virulence of endodontic pathogens
The virulence and pathogenicity of endodontic microorganisms in a biofilm state are enhanced by several factors.16-19 In 2010, Kishen listed basic mechanisms which allow endodontic pathogens to resist the commonly used root canal irrigants and medicaments.20 These mechanisms are usually associated with the extracellular polymeric matrix, rate of bacterial growth, availability of nutrients and ability to adopt a resistant phenotype.20
The extracellular polymeric matrix can play a major role in increasing the resistance of endodontic biofilm against root canal irrigants and medicaments.16 Amongst the factors contributing to this resistance are: the ability of the extracellular polymeric matrix to facilitate adhesion of the biofilm structure to the tooth surface and provide mechanical stability to the biofilm. The matrix is a source of nutrition during starvation conditions.16 Moreover, the close proximity of the bacterial cells within the biofilm structure facilitates microbial communications such as exchange of genetic information and communication between cells (quorum sensing) in the regulation of gene expression and microbial synergy.16 Additionally, the extracellular polymeric matrix was shown to decrease the penetration rate of antimicrobial agents.21
Another factor associated with the high virulence of endo-dontic pathogens in a biofilm state is their ability to demonstrate dissimilar gene expression patterns compared with those microorganisms found in a planktonic state. As a result, microbial biofilm is found to be more resistant to antimicrobial agents.22,23
Antibacterial mechanisms of nanoparticles
The use of nanoparticles as antimicrobial agents has recently attracted considerable attention in the medical field as a result of their superior antibacterial properties compared with those of other antimicrobial agents together with a low potential to produce microbial resistance.24 The antimicrobial activity of nanoparticles against different microorganisms differs from that of its original bulk state24 and may vary according to the different types of nanoparticles.25
The efficacy of the nanoparticles to eliminate bacterial cells is attributed to the concurrent effect of two different mechanisms (Figure 1). One involves the binding of nanoparticles to the targeted bacterial cell membrane through electrostatic forces, causing an alteration in the membrane potential, depolarization and eventually loss of membrane integrity.26 This results in disturbance of major bacterial cell functions such as respiration, transportation of nutrients and disturbance of energy transduction, leading subsequently to bacterial cell death.26 The second mechanism includes the production of oxygen free-radicals such as reactive-oxygen species (ROS) that can influence survival of the bacterial cell by blocking the protein function, destroying DNA and resulting in excess radical production.27
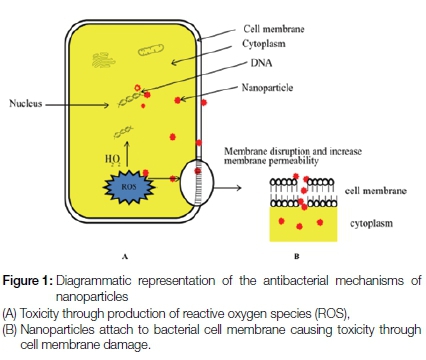
Antimicrobial efficacy of nanoparticles in endodontics
Different types of nanoparticles have been investigated recently in different forms in in vitro studies to evaluate their efficacy against endodontic pathogens.1,28,29 The nanoparticles used in these studies broadly can be classified into three categories according to their nature: metallic or inorganic, polymeric and bioactive non-organic nanoparticles.
Antibacterial efficacy of metallic or inorganic nano-particles
The antibacterial effect of metallic or inorganic nanoparticles such as silver, magnesium and zinc oxide against endodontic pathogens have been evaluated in many in vitro studies.30-32 Among these, the antibacterial effect of silver nanoparticles was the most commonly considered in the literature.
The antimicrobial properties of silver nanoparticles were first demonstrated by Jose Ruben et al.33Silver nanoparticles have the ability to bind to the negatively charged part of the bacterial cell membrane, disturbing its functions such as permeability and respiration, causing leaking of the cytoplas-mic content and eventually rupture of the bacterial cell. As a result, the nanoparticles will infiltrate inside the cytoplasmic content and interact with sulphur- and phosphorus- containing proteins such as DNA and RNA, causing further damage to the bacterial cell.33 Additionally, the silver nanoparticles release silver ions when in contact with an aqueous media, further disturbing the bacterial functions.33-37
Wu et al. evaluated the effect of silver nanoparticles in a concentration of 0.1% as an endodontic irrigant solution and as a gel in two different concentrations (0.02% and 0.1%) against Enterococcus faecaíis biofilm.31 The solution did not cause any major change to the structure of E. faecaíis biofilm. However, the use of silver nanoparticles in a gel form with a concentration of 0.02% had the ability to disrupt the structural integrity of the E. faecaíis biofilm more than a 0.01% silver nanoparticle gel and thus decreased the number of viable bacteria.31
The antibacterial effect of silver nanoparticles as an intra-canal medicament in a paste form was evaluated by Buruniera et al.38Three different carriers for silver nanoparticles were used in their study, namely; hydroxyethylcellulose polymer, carbomer polymer gel and polyethylene glycol. The antibacterial efficacy of these new materials was evaluated against different bacterial species such as E. faecaíis, Pseudomonas aruginosa, Streptococcus mutans, E. coli and Staphylococcus aureus. This study showed that the use of silver nanoparticles when loaded into different types of carriers had an antibacterial effect against the tested bacterial species. Additionally, the use of hydroxyethylcellulose polymer gel as a vehicle for silver nanoparticles provided the maximum homogeneity and fluidity as a carrier compared with the other materials and thus resulted in improved antibacterial properties.38
Silver nanoparticles may hold different surface charges and the effect of these variations on their antibacterial efficacy was evaluated by Abbaszadegan et al.39The efficacy of three preparations having surface charges of neutral, negatively-charged or positively-charged against planktonic cells of E. faecaíis was compared with that of sodium hypochlorite and Chlorhexidine. Positively-charged silver nanoparticles showed a minimal effect against the tested bacterial species. However, unlike with neutral and negatively-charged silver nanoparticles, sodium hypochlorite and chlorhexidine, the minimal antibacterial effect was still shown with the positively charged silver nanoparticles at lower concentrations. Additionally, some tissue inhibitors, such as dentine powder or the remnants of pulp tissue, that have the ability to inhibit the antibacterial effect of root canal medicaments,40 were shown to have no such effect on the antibacterial properties of the positively-charged silver nanoparticles even after 24 hours contact time. The study concluded that the antibacterial effects of different surface-charged silver nanoparticles, sodium hypochlorite, and chlorhexidine depended on their concentrations and the contact time.39
Furthermore, silver nanoparticles were shown to enhance the antibacterial properties of some intra-canal medicaments such as calcium hydroxide, as has been demonstrated by Afkhami et al. in their study which tested the effect of the combination on E. faecalis.41
The use of silver nanoparticles as an antimicrobial agent against endodontic pathogens shows promise. However, further investigation is required to evaluate any effect on the colour stability of the tooth structure, the dentine surface and possible cytotoxic actions on human cells.
Magnesium-containing nanoparticles (Mg-NPs)
Magnesium-containing nanoparticles were suggested for use as antimicrobial agents against endodontic pathogens due to their known antibacterial properties against gram-positive and gram-negative bacteria, spores and viruses.25 Magnesium-containing nanoparticles are either magnesium-oxide nanoparticles or magnesium-halogen-containing nanoparticles such as chlorine, bromine and fluorine.26,42 The antimicrobial properties of magnesium-containing-nanoparticles were thought to be due to multiple mechanisms. Similar to the common antimicrobial mechanisms of nanoparticles, magnesium-halogen-containing nanoparticles infiltrate inside the bacterial cell, resulting in a disturbance in the membrane potential. The penetration facilitated the DNA binding and lipid peroxidation effects of the nanoparticles, causing more destruction of the bacterial cell.43 Magnesium-oxide nanoparticles were found to be bactericidal when present in an aqueous form as a result of the action of superoxide anions that formed on the bacterial cell surface.44
The antibacterial efficacy of different concentrations of magnesium oxide nanoparticles (5 mg/L and 10 mg/L) and 5.25% sodium hypochlorite and 2% chlorhexidine against endodontic pathogens such as E. faecalis, S. aureus and Candida albicans was studied by Monzavi et al.30 The results showed no significant differences in the antimicrobial efficacies of the irrigant solutions used against the tested endodontic pathogens. However, the inclusion of magnesium oxide nanoparticles in an irrigant solution produced extended antibacterial activity when compared with sodium hypochlorite.30
Zinc oxide nanoparticles (ZnO-NPs)
Zinc oxide nanoparticles showed high antibacterial ef-fectiveness,45,46 destroying microbial cells in a higher pH environment.47 The antibacterial mechanism of zinc oxide nanoparticles is similar to that of other types of nanoparti-cles, causing increased permeability of the cell wall membrane, a release of cytoplasmic content and cell death.48 The bactericidal effect of zinc oxide nanoparticles was shown to be related to size, the smaller the size the higher the antibacterial effect and the production of reactive oxygen species such as hydrogen peroxide when in contact with an aqueous medium.42,47,49-51 Additionally, zinc oxide nanoparticles can produce zinc ions inside the bacterial cell causing disturbances in its enzymatic system and the mechanism of amino acid metabolism, resulting in further damage.52 The antibacterial effect of zinc oxide nanoparti-cles has been shown to depend on concentration, higher levels resulting in the maximum antibacterial effect.47
The antibacterial and antibiofilm efficacy of zinc oxide na-noparticles against some endodontic pathogens such as E. faecalis were assessed by Kishan et al.32It was shown that zinc oxide nanoparticles can reduce the colony forming units of E. faecalis in a biofilm state. The same antibacterial effect was evident when zinc oxide nanoparticles were incorporated into a resin based root canal sealer. Also shown was a 95% reduction in the ability of E. faecalis to adhere and form biofilm in a dentinal wall.32 Another study found that the thickness and structure of the E. faecalis biofilm was reduced and disrupted after 72 hours contact time with zinc oxide nano-particles but concluded that zinc oxide nanoparticles have the ability to eliminate E. faecalis in a planktonic state but not in a biofilm state.29 Varying degrees of antibacterial effects against P. aeruginosa, E. faecalis, C. albicans, S.aureus and Kocuria rhizophila were shown when zinc oxide nanopar-ticles were incorporated into polyethylene glycol to form a creamy mix and used as an intra-canal medicament.28
Several studies also shown the antibacterial effect of using metallic nanoparticles against endodontic pathogens (Table 1). However, further developments in understanding their chemical structure are required if their antimicrobial properties are to be enhanced and further clinical testing of the antibacterial effects should be undertaken.
Application of polymeric nanoparticles in endodontics
Chitosan nanoparticles
Polymeric nanoparticles gained significant interest amongst researchers as a result of their biocompatible and antimicrobial properties.53 Chitosan nanoparticles (Cs-NPs) are one of the commonly investigated polymeric nanoparticles in en-dodontics. Chitosan is a natural polysaccharide54 that is obtained by deacetylation of chitin,55 one of the most abundant polysaccharides in nature that forms most of the external skeleton of arthropods such as crabs and shrimps.56 Chemically, chitin is composed of (1-4)-linked 2-acetamido-2-deoxy-ß-D-glucose.57 A modification in the structure of chitin in which the acetyl group is reduced by 40% to 35% by chemical hydrolysis in alkaline solution and high temperature produces a new chemical formula that consists of a copolymer of (1-4)-2-amine-2-deoxy-ß-D-glucan and (1-4)-2-acetamide-2- deoxy-ß-D-glucan which is known as chitosan.58
Although the antimicrobial efficacy of chitosan was assessed by several investigators, the antibacterial mechanism of chitosan has not yet been fully clarified. Several hypotheses have been postulated, based on its cationic nature.59 Chitosan with low molecular weight was considered to have the ability to penetrate the bacterial cell membrane and then to bind to the DNA, inhibiting its transcription and mRNA synthesis, while chitosan with high molecular weight was surmised to bind to the negatively charged components of the bacterial cell wall, forming an impermeable layer and blocking transportation into the cell.60 Another alternative hypothesis for the antibacterial mechanism of chitosan is thought to be as a result of its ability to bind to the negatively charged bacterial cell membrane, increasing its permeability and ultimately resulting in leaking of the cytoplasmic contents and bacterial cell death.61 Others postulated that as chitosan has the ability to chelate metals microbial growth was inhibited by reducing enzyme activity through metal chelation.62
In endodontics, the use of chitosan nanoparticles as an antimicrobial agent was investigated against some endo-dontic pathogens (Table 2). Kishan et al.32and Shertha et al.29showed that chitosan nanoparticles can completely eliminate E. faecalis pathogens present in a planktonic state, and can cause a significant reduction of bacteria in the biofilm state.29,32
Chitosan nanoparticles can be used as a drug carrier.59 This property was utilized by Shertha and Kishan by conjugating a photosensitizer material (rose bengal) to the chitosan structure and then evaluating its antimicrobial property against biofilms of E. faecalis, Streptococcus oralis, Prevotella. intermedia, and Actinomyces naeslundi.63They showed that such a conjugation can destroy the bacterial cell membrane of the tested bacterial species and then can penetrate deep into the biofilm structure of the tested species reducing the biofilm thickness and the number of microbial cells.63
The presence of some tissue factors such as dentine powder, dentine matrix and remnants of pulp tissue within the root canal system was shown to inhibit the antimicrobial properties of some endodontic disinfectants.64,65 The effect of these tissue factors was evaluated by Shertha and Kishan against the antimicrobial properties of synthesized chitosan nanoparticles conjugated with rose bengal as photosensitizer.66 Remnants of bacterial tissue and dentine powder reduced the antibacterial efficacy of the conjugated solution in the first few hours. However, complete elimination of the tested bacteria before and after application of low energy photodynamic light was shown after 24 hours.66
Chitosan nanoparticles were incorporated into a zinc-oxide eugenol based sealer and were assessed for their antibacterial effect against E. faecalis biofilm on bovine root dentine treated by phosphorylated chitosan, chitosan conjugated with rose bengal and a combination of phos-phorylated chitosan and chitosan conjugated with rose bengal, respectively. There was an inhibition of E. faecalis biofilm formation, the degree of inhibitory effects varying with the different treatment solutions used.67
Bioactive glass nanoparticles
In 1971 a new material with antibacterial properties and that can bond to the bone structure was developed. The developed material consisted of 45% SiO2, 24% Na2O, 24.5% CaO and 6% P2O5 and was named Bioglass.68 The antimicrobial property of bioactive glass material was shown to be through its ability to: [i] release its ions when it came into contact with an aqueous medium, [ii] increase the surrounding pH [iii] increase the osmotic pressure around the bacterial cell causing inhibition of bacterial growth and [iv] to precipitate calcium and phosphate ions in the bacterial cell membrane, disturbing its functions.69 The use of 45S5 bioactive glass nanoparticles was found to produce better antibacterial effects against E. faecalis than micro-sized bioactive glass particles.70 However, Zehnder et al showed that calcium hydroxide is more effective than 20 - 60 nm sized bioactive glass nanoparti-cles against E. faecaíis.71
The use of bioactive glass nanoparticles as an antimicrobial agent as a replacement for the commonly used endo-dontic disinfectants is still an area of controversy as a result of the variation in results obtained by different studies regarding their efficacy against endodontic pathogens. More studies after further improvement in the synthesis of bioactive glass nanoparticles are needed.
DISCUSSION
The large number of nanoparticle materials available today provide multiple choices for their use in in the medical field. Current endodontic research is focused on evaluating the antimicrobial properties of some nanoparticles as new agents against endodontic pathogens. Available studies show that there is promise in the use of different types of nanoparticles as antimicrobial agents especially against persistent endodontic pathogens such as E. faecalis. Whilst it appears that some of the shortcomings, of traditional root canal irrigants and medicaments can be overcome, more in-vitro and in-vivo studies are needed to evaluate which nanoparticles are more appropriate for use as a root canal irrigant solution, intra-canal medicament, or even bioactive root canal filling material, which is still an area of further investigation. Also, the antimicrobial effects of the nanoparticles need to be tested against a large variety of persistent endodontic pathogens. Indeed, more studies are needed to evaluate the biocompatibility, safety, cost and ease of use of these innovative materials.
ACRONYMS
ROS: reactive-oxygen species
References
1. Bhardwaj A, Bhardwaj A, Misuriya A, Maroli S, Manjula S, Singh AK. Nanotechnology in dentistry: Present and future. Journal of International Oral Health 2014;6:121. [ Links ]
2. Feynman RP. There's plenty of room at the bottom. Engineering and Science. 1960;23:22-36. [ Links ]
3. Sanchez F, Sobolev K. Nanotechnology in concrete-a review. Construction and Building Materials 2010;24:2060-71. [ Links ]
4. Commission E. Commission Recommendation of 18 October 2011 On The Definition of Nanomaterial (2011/696/EU). Official Journal of the European Communities: Legis 2011. [ Links ]
5. Tiwari JN, Tiwari RN, Kim KS. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Progress in Materials Science 2012;57:724-803. [ Links ]
6. Mantri SS, Mantri SP. The nano era in dentistry. Journal of Natural Science, Biology, and Medicine 2013;4:39. [ Links ]
7. Neel EAA, Bozec L, Perez RA, Kim H-W, Knowles JC. Nanotechnology in dentistry: prevention, diagnosis, and therapy. International Journal of Nanomedicine 2015;10:6371. [ Links ]
8. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surgery, Oral Medicine, Oral Pathology 1965;20:340-9. [ Links ]
9. Sundqvist G, Figdor D. Life as an endodontic pathogen. Endodontic Topics 2003;6:3-28. [ Links ]
10. Gomes BPFA, Pinheiro ET, Gadê-Neto CR, et al. Microbiological examination of infected dental root canals. Oral Microbiology & Immunology 2004;19:71-6. [ Links ]
11. Jhajharia K, Parolia A, Vikram Shetty K, Mehta LK. Biofilm in endodontics: a review. Journal of International Society of Preventive & Community Dentistry 2015;5:1-12. [ Links ]
12. Siqueira JF, Rögas IN, Ricucci D. Biofilms in endodontic infection. Endodontic Topics 2010;22:33-49. [ Links ]
13. Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. Journal of Endodontics 1987;13:29-39. [ Links ]
14. Mohammadi Z, Palazzi F, Giardino L, Shalavi S. Microbial biofilms in endodontic infections: an update review. Biomedical Journal 2013;36:59. [ Links ]
15. Kokare C, Chakraborty S, Khopade A, Mahadik K. Biofilm: Importance and applications. Indian Journal of Biotechnology 2009;8:159-68. [ Links ]
16. Flemming H-C, Wingender J. The biofilm matrix. Nature Reviews Microbiology 2010;8:623-33. [ Links ]
17. Tronstad L, Sunde PT. The evolving new understanding of endodontic infections. Endodontic Topics 2003;6:57-77. [ Links ]
18. Siqueira JF, Rögas IN. Present status and future directions in endodontic microbiology. Endodontic Topics 2014;30:3-22. [ Links ]
19. Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nature Reviews Microbiology 2010;8:471-80. [ Links ]
20. Kishen A. Advanced therapeutic options for endodontic biofilms. Endodontic Topics 2010;22:99-123. [ Links ]
21. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318-22. [ Links ]
22. Beloin C, Valle J, Latour-Lambert P, et al. Global impact of mature biofilm lifestyle on Escherichia coli K12 gene expression. Molecular Microbiology 2004;51:659-74. [ Links ]
23. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology 2002;184:1140-54. [ Links ]
24. Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. International Journal of Nanomedicine 2012;7:2767. [ Links ]
25. Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evidence-Based Complementary and Alternative Medicine 2015;2015:16. [ Links ]
26. Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Advanced Drug Delivery Reviews 2013;65:1803-15. [ Links ]
27. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology 2007;39:44-84. [ Links ]
28. Guerreiro-Tanomaru JM, Figueiredo Pereira K, Almeida Nascimento C, Basso Bernardi MI, Tanomaru-Filho M. Use of nano- particulate zinc oxide as intracanal medication in endodontics: ph and antimicrobial activity. Acta Odontológica LatinoAmericana 2013;26:167-72. [ Links ]
29. Shrestha A, Zhilong S, Gee NK, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. Journal of Endodontics 2010;36:1030-5. [ Links ]
30. Monzavi A, Eshraghi S, Hashemian R, Momen-Heravi F. In vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogens. Clinical Oral Investigations 2015;19:349-56. [ Links ]
31. Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. Journal of Endodontics 2014;40:285-90. [ Links ]
32. Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. Journal of Endodontics 2008;34:1515-20. [ Links ]
33. Jose Ruben M, Jose Luis E, Alejandra C, et al. The bactericidal effect of silver nanoparticles. Nanotechnology 2005;16:2346. [ Links ]
34. Abbasi E, Milani M, Fekri Aval S, et al. Silver nanoparticles: synthesis methods, bio-applications and properties. Critical Reviews in Microbiology 2014:1-8. [ Links ]
35. Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International Nano Letters 2012;2:1-10. [ Links ]
36. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram negative bacteria. Journal of Colloid and Interface Science 2004;275:177-82. [ Links ]
37. Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environmental Science & Technology 2010;44:5649-54. [ Links ]
38. Bruniera JFB, Silva-Sousa YTC, Lara MG, Pitondo-Silva A, Marcaccini AM, Miranda CES. Development of intracanal formulation containing silver nanoparticles. Brazilian Dental Journal 2014;25:302-6. [ Links ]
39. Abbaszadegan A, Nabavizadeh M, Gholami A, et al. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: a promising disinfectant in root canal treatment. International Endodontic Journal 2015;48:790-800. [ Links ]
40. Siqueira JF, Rögas IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. Journal of Endodontics 2008;34:1291-301. e3. [ Links ]
41. Afkhami F, Pourhashemi SJ, Sadegh M, Salehi Y, Fard MJK. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. Journal of Dentistry 2015;43:1573-9. [ Links ]
42. Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence 2011;2:395- 401. [ Links ]
43. Lellouche J, Kahana E, Elias S, Gedanken A, Banin E. Anti-biofilm activity of nanosized magnesium fluoride. Biomaterials 2009;30:5969-78. [ Links ]
44. Huang L, Li D-Q, Lin Y-J, Wei M, Evans DG, Duan X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. Journal of Inorganic Biochemistry 2005;99:986-93. [ Links ]
45. Liu Y, He L, Mustapha A, Li H, Hu Z, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. Journal of Applied Microbiology 2009;107:1193-201. [ Links ]
46. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters 2015;7:219-42. [ Links ]
47. Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. International Journal of Inorganic Materials 2001;3:643-6. [ Links ]
48. Huang Z, Zheng X, Yan D, et al. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 2008;24:4140-4. [ Links ]
49. Jalal R, Goharshadi EK, Abareshi M, Moosavi M, Yousefi A, Nancarrow P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Materials Chemistry and Physics 2010;121:198-201. [ Links ]
50. Zhang L, Ding Y, Povey M, York D. ZnO nanofluids - a potential antibacterial agent. Progress in Natural Science 2008;18:939-44. [ Links ]
51. Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Science and Technology of Advanced Materials 2008;9:035004. [ Links ]
52. Song W, Zhang J, Guo J, et al. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicology Letters 2010;199:389-97. [ Links ]
53. Virlan MJR, Miricescu D, Radulescu R, et al. Organic nanomaterials and their applications in the treatment of oral diseases. Molecules 2016;21:207. [ Links ]
54. §enel S, Ikinci G, Ka§ S, Yousefi-Rad A, Sargon MF, Hincal AA. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. International Journal of Pharmaceutics 2000;193:197-203. [ Links ]
55. Silva PV, Guedes DFC, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: a new solution for removal of smear layer after root canal instrumentation. International Endodontic Journal 2013;46:332-8. [ Links ]
56. Rinaudo M. Chitin and chitosan: properties and applications. Progress in Polymer Science 2006;31:603-32. [ Links ]
57. Park BK, Kim M-M. Applications of chitin and its derivatives in biological medicine. International Journal of Molecular Sciences 2010;11:5152-64. [ Links ]
58. Goy RC, Britto Dd, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros 2009;19:241-7. [ Links ]
59. Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. International Journal of Food Microbiology 2010;144:51-63. [ Links ]
60. Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003;4:1457-65. [ Links ]
61. Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research 2004;339:2693-700. [ Links ]
62. Cuero R, Osuji G, Washington A. N-carboxymethylchitosan inhibition of aflatoxin production: role of zinc. Biotechnology Letters 1991;13:441-4. [ Links ]
63. Shrestha A, Kishen A. Antibiofilm efficacy of photosensitizer- functionalized bioactive nanoparticles on multispecies biofilm. Journal of Endodontics 2014;40:1604-10. [ Links ]
64. Haapasalo HK, Sirén EK, Waltimo TMT, Örstavik D, Haa- pasalo MPP. Inactivation of local root canal medicaments by dentine: an in vitro study. International Endodontic Journal 2000;33:126-31. [ Links ]
65. Haapasalo M, Qian W, Portenier I, Waltimo T. Effects of dentin on the antimicrobial properties of endodontic medicaments. Journal of Endodontics 2007;33:917-25. [ Links ]
66. Shrestha A, Kishen A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. Journal of Endodontics 2014;40:566-70. [ Links ]
67. DaSilva L, Finer Y, Friedman S, Basrani B, Kishen A. Biofilm formation within the interface of bovine root dentin treated with conjugated chitosan and sealer containing chitosan nanoparticles. Journal of Endodontics 2013;39:249-53. [ Links ]
68. Hench LL. The story of Bioglass®. Journal of Materials Science: Materials in Medicine 2006;17:967-78. [ Links ]
69. Stoor P, Söderling E, Salonen JI. Antibacterial effects of a bio-active glass paste on oral microorganisms. Acta Odontologica Scandinavica 1998;56:161-5. [ Links ]
70. Waltimo T, Brunner T, Vollenweider M, Stark W, Zehnder M. Antimicrobial effect of nanometric bioactive glass 45S5. Journal of Dental Research. 2007;86:754-7. [ Links ]
71. Zehnder M, Luder HU, Schätzle M, Kerosuo E, Waltimo T. A comparative study on the disinfection potentials of bioactive glass S53P4 and calcium hydroxide in contra-lateral human premolars ex vivo. International Endodontic Journal. 2006;39:952-8. [ Links ]
72. Mohn D, Zehnder M, Imfeld T, Stark WJ. Radio-opaque nanosized bioactive glass for potential root canal application: evaluation of radiopacity, bioactivity and alkaline capacity. International Endodontic Journal. 2010;43:210-7. [ Links ]
 Correspondence:
Correspondence:
DS Moodley
Department of Restorative Dentistry, Faculty of Dentistry, University of the Western Cape
Private Bag X1, Tygerberg, 7505
Cape Town, South Africa
Tel: +27 21 9373090
E-mail: dmoodley@uwc.ac.za














