Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.72 no.2 Johannesburg Mar. 2017
RESEARCH
Xerostomia and salivary flow rates in HIV patients
CherianI; A JefthaII
IBDS, PG Dip Dent (Clinical Dentistry), MSc (Dent). Empilweni Gompo CHC, Buffalo City Metro, East London, Department of Health, Eastern Cape
IIBChD, MChD (Oral Medicine and Periodontology). Department Oral Medicine and Periodontology, University of the Western Cape
SUMMARY
INTRODUCTION: Whilst the incidence of oral manifestations in HIV infected patients has decreased with the advent of highly active antiretroviral therapy (HAART), salivary gland disease is reported to be increasing among those on this treatment regime.
AIMS AND OBJECTIVES: To compare the prevalence of xerostomia and mean salivary flow rates in three groups: HIV negative (Gr-1), HIV positive but not on HAART (Gr-2) and HIV positive on HAART (Gr-3).
DESIGN: A cross sectional analytical study.
METHODS: Xerostomia was assessed using a questionnaire. Saliva was collected and flow rates established. CD4 counts, viral loads and HAART regimens were recorded where appropriate.
RESULTS: Significant differences were observed between the groups regarding the prevalence of xerostomia (p=0.006), mean resting (p=0.010) and stimulated (p=0.034), salivary flow rates. Gr-2 showed the greatest salivary deficiency. Salivary flow was not decreased by HAART. Levels of CD4 <350 were linked to low resting flow rates in Gr-2. In Gr-3, patients on fixed dose combination (FDC) showed a significantly lower stimulated flow rate (p=0.034) than those on other HAART regimens.
CONCLUSION: HIV positive patients not on HAART are more vulnerable to decreased salivary flow rates. HAART did not adversely affect xerostomia or salivary flow rates in this population group.
INTRODUCTION
It is reported that South Africa has the largest population in the world of persons living with HIV. The total number of infected persons has been estimated to be 6.4-million (12.2% of the population).1 This marked prevalence had been attributed by the Human Sciences Research Council (HSRC) to the combined effects of new infections and the success of an expanded ART programme, which had increased survival rates among HIV-infected individuals.1
Although HIV had been associated with a variety of oral opportunistic lesions during the early days of its emergence, the incidence of these lesions has decreased in patients on HAART.2-5 In contrast, HIV-associated Salivary Gland Disease (HIV-SGD) in general seemed to be slowly increasing in prevalence during the HAART era.6-12 Reduced salivary flow and xerostomia have been reported with the use of HAART.8-10
The most common salivary gland changes reported were those related to saliva production, manifesting as hy-posalivation and xerostomia, the perceived feeling of a dry mouth, which may or may not be associated with salivary gland hypofunction. It is subjective and can be measured by means of questionnaires13 or visual analogue scales.14
Hyposalivation on the other hand, is a demonstrable reduction in salivary flow rate that can be measured objectively by collecting saliva over a specified period of time.15 Often these terms have been used interchangeably but studies have demonstrated that xerostomia may not necessarily indicate an actual measurable reduction in salivary flow rate. The reverse is also true, as some patients with reduced flow rates did not complain of xerostomia.13
The functions of saliva include lubrication, buffering capacity, tooth remineralisation and antimicrobial and antifungal protection. A reduction in the flow rates of saliva would adversely affect these vital functions resulting in an increase in dental caries, certain oral infections and a general oral discomfort. Salivary gland hypofunction has been shown to have a high predictive value for recurrent candidial infection.16 Denture wearers with low salivary flow rates have low denture retention. Busato et al.17concluded in their study that xerostomia further reduces the quality of life of people living with HIV and AIDS.
AIM
This study evaluated and compared the prevalence of self-reported xerostomia and the mean salivary flow rates in three patient groups: HIV positive patients on long term HAART, HIV positive patients not on HAART and HIV negative patients.
DESIGN AND METHODS
This was a cross-sectional analytical study. Ethical approval was obtained from the Senate Research Committee, University of Western Cape. Permission to conduct the study was obtained from the Department of Health, Eastern Cape.
Adult patients (18-55 years of age) who attended the HIV Counselling and Testing centre (HCT) and the Anti-retroviral (ARV) section at a public health care facility in East London, South Africa, were invited to participate in the study by written informed consent. The sample size was 150, with 50 individuals in each group. HIV negative patients were allocated to Gr-1. The HIV positive patients were divided based on treatment. Gr-2 included those who were HAART naive and Gr-3, those who had been on HAART for two years or more.
The exclusion criteria included patients who were acutely ill, those on any medication (other than HAART) that had a side effect of xerostomia, those diagnosed with any auto-immune salivary gland disease, and those who had received any head and neck radiation. Pregnant patients were excluded from the study. Totally edentulous patients were also excluded.
Data collection and procedure:
The incidence and the expression of xerostomia were evaluated based on the responses to a questionnaire which had been proposed by Sreebny and Valdini13 and which included only four questions:
1. Does your mouth usually feel dry?
2. Do you regularly do things to keep your mouth moist?
3. Do you get out of bed at night to drink fluids?
4. Does your mouth usually become dry when you speak?
These four questions were found by Sreebny and Valdi-ni to have a high specificity and predictive value.13 A positive response to any was considered as indicative of xerostomia.
Subjects refrained from eating and drinking 90 minutes before saliva collection. Saliva was collected in sterile plastic tubes through a funnel (Figure 1). A countdown timer was used to mark time elapsed.

Unstimulated whole-mouth saliva was collected by the "spitting method"15 into the tube for 3 minutes. At the end of three minutes, the tubes were collected and new tubes used for collecting chewing-stimulated saliva. A 2cm piece of sterile rubber was used for chewing and saliva collected by the same method. A metronome was used to regulate chewing to 45 strokes per minute. The subjects were asked to chew for one minute and spit the accumulated saliva into the tube. The rubber piece was kept in the mouth of the subject and the process was repeated two more times. All saliva samples collected were weighed on a calibrated scale and the flow rate per minute was calculated. Since the specific gravity of saliva is one, 1 gram is considered equivalent to 1 ml.
Statistical analysis
Data was captured on Microsoft Excel. Data analysis was done with the statistical software "R" version 2.15.0 (201203-30) (Copyright © 2012, The R Foundation for Statistical Computing). The data collected was subjected to descriptive analysis and prevalence was calculated. The significance of the differences of prevalence were calculated by Chi-squared test and, where applicable, Fischer's exact test. Statistical significance was set at p-value <0.05.
The influence of independent variables such as gender, age, CD4 count, smoking, viral load and medications on the prevalence of xerostomia was examined, using logistic regression. The differences in mean flow rates between the groups were examined by least squared linear regression and associated analysis of variance (ANOVA). The Student's t- test was used whenever differences between two mean flow rates needed to be analysed for significance.
RESULTS
The demographic and clinical data collected were as shown in Tables 1 and 2. The mean age of the sample was 34yrs with Gr-3 having a slightly higher mean age of 39 and Gr-1 having a younger sample with mean age of 30yr. 73% of the patients in the study were female. The total number of smokers was 17 (11%) with 8, 7 and 2 in Gr-1, Gr-2 and Gr-3 respectively. Of these only 2 (1%) patients, both in Gr-1, smoked more than 10 cigarettes per day. The mean CD4 count in Gr-2 was 339 and in Gr-3 it was 577. Six of the patients in Gr-2 and twelve in Gr -3 were on either Co-trimoxazole or INH or on both. In Gr-3, 34 of the 50 were on FDC (Fixed dose combination) and 16 were on other HAART regimens. Viral loads were available only for Gr-3 and 78% (39) of this group had a value lower than the detectable level (LTDL) so the effect of viral loads on the outcome measures were not calculated.

The influence of covariates on the prevalence of xerostomia was examined by fitting generalized linear models with the dependent variable xerostomia and the independent variables group, age, gender, smoking, CD4 and use of Co-trimoxazole and/or INH (using only Gr-2 and Gr-3). Multiple regression analysis revealed that for xerostomia, the significant predictor besides group was age, which was found to have a significant negative correlation at p=0.002. When mean flow rates were analysed, it was seen that although males generally had a higher mean flow rate, a significance was seen only for chewing- stimulated flow rate p=0.031.
A summary of outcome measures and the comparison thereof between the groups can be seen in Table 3. Significance was found between the groups for all relationships.

The overall prevalence of xerostomia was 50% in the total study population. Gr-2 showed the highest prevalence with 33 (66%) out of 50 patients responding affirmative to at least one of the xerostomia questions in the questionnaire. 25 (50%) patients and 17 (34%) patients, in Gr-1 and Gr-3 respectively, responded similarly. The differences in prevalence between the groups were examined using the Chi- Squared test. The hypothesis of homogeneous prevalence was rejected at level 0.006. The difference between Gr-2 and Gr-3 was found to be significant at p=0.002. Although Gr-2 had a higher xerostomia prevalence than the HIV negative patients in Gr-1, the difference between the two prevalences was not significant at p= 0.156.
The mean resting and chewing-stimulated salivary flow rates for each group was subjected to a one way Analysis of Variance (ANOVA). There was a significant difference for both flow rates between the groups as seen in Table 3.
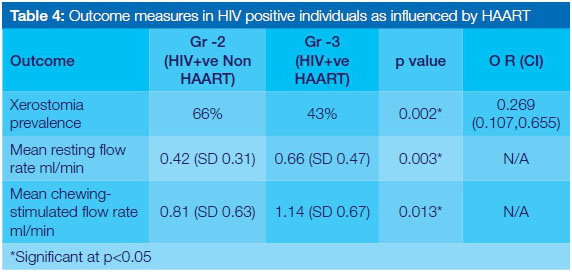
When the outcome measures were compared between the two HIV positive groups, similar results were seen (Table 4). Patients in Gr-2 had a higher prevalence of xerostomia (p=0.002) and a significant reduction in both resting and chewing-stimulated mean flow rates when compared with those in Gr-3. The difference in the mean flow rates between Gr-2 and Gr-3 was significant with p=0.003 for resting flow rate and p=0.013 for chewing-stimulated flow rates.
The influence of low CD4 (<350) on the outcome measures were analysed in both the HIV positive groups (Gr-2 and Gr-3) separately and together, irrespective of HAART. When mean flow rates of all the HIV positive patients were compared, although the flow rates were reduced in those with CD4 counts <350 cell/mm3, there was no statistical significance (Table 5). The difference in mean resting flow rates came close to significance at p= 0.055. When analysed separately, the mean resting flow rate was found to be significantly influenced by a low CD4 count only in Gr-2 (p=0.035).

In Gr-3 68% (n=34) were on FDC (which contains two NRTI's and one NNRTI). There was a non-significant increase in the prevalence of xerostomia and a significant reduction in the mean chewing-stimulated flow rate for those on FDC (p= 0.034) when compared with those on other HAART regimens (Figure 2).

DISCUSSION
A decrease in salivary function and a significant increase in xerostomic symptoms were noted among those who were not on HAART, ie Gr-2. The difference was greater for resting flow rate than for chewing-stimulated flow rate. During the resting phase, 65% unstimulated saliva is produced by the submandibular gland, 20% by the parotid, 7-8% from the sub-lingual and the remaining from minor salivary glands.18 According to Atkinson et al.19the function of the submandibular gland is affected earlier during the progression of HIV infection and the parotid glands are affected over a greater time.
While low CD4 counts (<200 cell/mm3) have been attributed by many authors to being a significant risk factor for xerostomia and hyposalivation,9,20,21 others did not find this correlation significant.10,22,23 According to Schiødt et al.,24the reduction in salivary flow is "likely to be a function of the degree of inflammatory infiltrate in the gland but not associated with degree of immune deficiency". In the current study, a CD4 count < 350 was used as the criterion for a low value since this is the reference level used by the South African Public Health system at which HAART was initiated at the time of the study.
Xerostomia prevalence did not show a significant difference between the two CD4 groups. The mean resting flow rate for those with CD4 counts <350 cell/mm3, when calculated in Gr-2 alone, was found to be significant at p= 0.035 (Table 5). This further points to the oral health vulnerability of these HIV positive patients with a low CD4 count in whom HAART is yet to be initiated and during the period while waiting for the therapeutic effect of HAART to improve the CD4 count. Low CD4 counts in patients on HAART, Gr-3, did not seem to affect mean resting flow rates.
More than 50% of the saliva produced under stimulation is from the parotid gland.18 Although the exact mechanism and long term effects of HAART on the parotid is unknown, lym-phocytic infiltration, accinar changes, lipomatous changes and immune reconstitution inflammatory syndrome (IRIS) have all been proposed.6,9,25 When comparing the outcomes of those on FDC with those on other HAART regimens, there was a statistically insignificant increase in the prevalence of xerostomia. But a significant reduction was seen in the mean chewing-stimulated flow rate for those on FDC. The patients on FDC were further separated based on duration of time on FDC as <3month, 3-6 months and >6 months. Further evaluation of their mean flow rates showed progressive improvement in both resting and chewing flow rates as the duration on FDC increased.
This could be due to the fact that of the 34 patients that were on FDC, 32 had just switched their HAART regimen in the past six months. Silverberg et al.,26and Navazesh et al.,21had found in their study that patients on stable HAART usage had higher salivary flow rates and lesser xerostomia complaints than those that switched HAART or had discontinued treatment in the previous six months.
Interestingly, age was negatively co-related to xerostomia unlike many other reports that associated increasing age with increasing xerostomia complaints.13,27,28 The range of ages included in the study; 18-55 years, do not significantly affect xerostomia27 and this contradictory influence could be coincidental due to other systemic factors. The fact that in this study, Gr-3 had the oldest mean age yet the group had the lowest prevalence for xerostomia and the highest mean flow rates, might have influenced this result.
CONCLUSION
Salivary gland dysfunction was observed more readily in those who were immuno-compromised and not yet on HAART. When planning an intense prophylactic treatment regimen, special attention should be paid to prevent and manage the oral conditions that are associated with reduced salivary flow in these individuals. HAART in itself did not appear to adversely affect xerostomic perceptions or salivary flow rate. The improved immunity that came from being on anti-retroviral treatment was beneficial to salivary gland function. Duration of HAART, change in regimen, type of regimen, all seem to have had an effect on salivary flow rate.21,23,26 Thus, studies on larger samples and of longitudinal design are necessary to explore the possibility of similar findings in the South African context. This would in turn further pin point those vulnerable to salivary hypofunc-tion and its effects, enabling timeous prophylactic actions.
The HIV prevalence in the Eastern Cape is estimated to be 11.6%.1 With HIV infections being managed progressively more successfully by the country's health care services, more and more HIV infected people are living longer and healthier lives while on HAART. Therefore, it is imperative that the general dental practitioner be aware of the salivary gland effects and resultant oral consequences seen in HIV infection and subsequent HAART.
ACRONYMS
ART: Anti-retroviral therapy
ARV: Anti-retroviral
FDC: Fixed dose combination
GR: Group
HAART: Highly Active Anti-Retroviral Therapy
HIV- SGD: HIV associated salivary gland disease
HCT: HIV Counselling and Testing
INH: Isoniazid, Iso-nicotinic acid hydrazide
NRTI: Nucleoside reverse transcriptase inhibitors
NNRTI: Non-Nucleoside reverse transcriptase inhibitors
References
1. Shisana O, Labadarios D, Rehle T, et al. SANHANES-1 Team (2013) South African National Health and Nutrition Examination Survey (SANHANES-1). Cape Town: HSRC Press.[Online] http://www.hsrc.ac.za/en/media-briefs/populationhealth/results-sanhanes1 [2013, 1 September]. [ Links ]
2. Greenspan D, Gange SJ, Phelan JA, et al. Incidence of oral lesions in HIV-1-infected women: reduction with HAART. J Dent Res. 2004 Feb; 83(2): 145-50. [ Links ]
3. Schmidt-Westhausen AM, Priepke F, Bergmann FJ, Reichart PA. Decline in the rate of oral opportunistic infections following introduction of highly active antiretroviral therapy. J Oral Pathol Med. 2000 Aug 1; 29(7): 336-41. [ Links ]
4. Masiiwa A, Naidoo S. Oral lesions in HIV-infected patients, before and after antiretroviral treatment: original research. South Afr J Epidemiol Infect. 2011 Jan 1; 26(4): 271-3. [ Links ]
5. Ortega KL, Vale DA, Magalhäes MH. Impact of PI and NNRTI HAART-based therapy on oral lesions of Brazilian HIV-infected patients. J Oral Pathol Med. 2009 Jul 1; 38(6): 489-94. [ Links ]
6. Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001 May 5; 357(9266): 1411-2. [ Links ]
7. Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ Jr. Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Mar 31; 89(3): 299-304. [ Links ]
8. Freeman AD, Liberali S, Coates EA, Logan RM. Oral health in Australian HIV patients since the advent of combination antiretroviral therapy. Aust Dent J. 2012 Dec1; 57(4): 470-6. [ Links ]
9. Navazesh M, Mulligan R, Barrón Y, et al. A 4-year longitudinal evaluation of xerostomia and salivary gland hypofunction in the Women's Interagency HIV Study participants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2003 June 30; 95(6): 693-8. [ Links ]
10. Nittayananta W, Talungchit S, Jaruratanasirikul S, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010 May; 39(5): 397-406. [ Links ]
11. Naidoo S, Chikte U. Oro-facial manifestations in paediatric HIV: a comparative study of institutionalized and hospital outpatients. Oral Dis. 2004 Jan 1; 10(1): 13-8. [ Links ]
12. Matee M, Scheutz F, Moshy J. Occurrence of oral lesions in relation to clinical and immunological status among HIV-infected adult Tanzanians. Oral Dis. 2000 Mar 1; 6(2):106-11. [ Links ]
13. Sreebny LM, Valdini A. Xerostomia. Part I: Relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol. 1988 Oct 1; 66(4): 451-8. [ Links ]
14. Pai S, Ghezzi EM, Ship JA. Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001 Mar 31; 91(3): 311-6. [ Links ]
15. Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992 Jul 1; 71(7): 1363-9. [ Links ]
16. McCarthy GM, Mackle ID, Koval J, Sandhu HS, Daley TD. Factors associated with increased frequency of HIV-related oral candidiasis. J Oral Pathol Med. 1991 Aug 1; 20(7): 332-6. [ Links ]
17. Busato IMS, Thomaz M, Toda AI, et al. Prevalence and impact of xerostomia on the quality of life of people living with HIV/AIDS from Brazil. Spec Care Dentist. 2013 May 1; 33(3):128-32. [ Links ]
18. De Almeida, Patricia Del Vigna, Gregio A, Machado M, De Lima A, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008 Mar 1; 9(3): 72-80. [ Links ]
19. Atkinson JC, Yeh C, Bermudez D, Fox PC, Baum BJ. Longitudinal evaluation of major salivary gland function in HIV-1 infected patients. J Oral Pathol Med. 1989 Sep 1; 18(8): 469-70. [ Links ]
20. Navazesh M, Mulligan R, Komaroff E, Redford M, Greenspan D, Pkelan J. The prevalence of Xerostomia and salivary gland hypofunction in a cohort of HIV-positive and at-risk women. J Dent Res. 2000 Jul 1; 79(7): 1502-7. [ Links ]
21. Navazesh M, Mulligan R, Karim R, et al. Effect of HAART on salivary gland function in the Women's Interagency HIV Study (WIHS). Oral Dis. 2009 Jan 1; 15(1): 52-60. [ Links ]
22. Pavithra S, Ranganathan K, Rao UK, Joshua E, Rooban T, Kumarasamy N. Impact of highly active antiretroviral therapy on salivary flow in patients with human-immuno deficiency virus disease in Southern India. J Oral Maxillofac Pathol. 2013 Jan 1; 17(1): 17-22. [ Links ]
23. López-Verdín S, Andrade-Villanueva J, Zamora-Perez AL, Bologna-Molina R, Cervantes-Cabrera JJ, Molina-Frechero N. Differences in salivary flow level, xerostomia, and flavor alteration in Mexican HIV patients who did or did not receive antiretroviral therapy. AIDS Res Treat. 2013 Dec 19; 2013:613278. [ Links ]
24. Schiødt M, Dodd CL, Greenspan D, et al. Natural history of HIV-associated salivary gland disease. Oral Surg Oral Med Oral Pathol. 1992 Sep 1; 74(3): 326-31. [ Links ]
25. Olive A, Salavert A, Manriquez M, Clotet B, Moragas A. Parotid lipomatosis in HIV positive patients: a new clinical disorder associated with protease inhibitors. Ann Rheum Dis. 1998 Dec; 57(12): 749. [ Links ]
26. Silverberg MJ, Gore ME, French AL, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women's Interagency HIV Study. Clin Infect Dis. 2004 Sep 1; 39(5): 717-24. [ Links ]
27. Flink H, Bergdahl M, Tegelberg Ä, Rosenblad A, Lagerlöf F. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008 Dec 1; 36(6): 523-31. [ Links ]
28. Nederfors T, Isaksson R, Mörnstad H, Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population-relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997 Jun 1; 25(3): 211-6. [ Links ]
 Correspondence:
Correspondence:
Anney P Cherian:
E-mail: anneyc@mweb.co.za














