Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.72 no.2 Johannesburg Mar. 2017
CLINICAL REVIEW
Human papillomavirus infection of the oral cavity: what the dentist should know
WFP van HeerdenI; EJ RaubenheimerII; BK BunnIII
IBChD, MChD, FC Path (SA)Oral Path, PhD, DSc. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria
IIBChD, MChD, FC Path (SA)Oral Path, PhD, DSc. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria and Ampath Laboratories, Pretoria
IIIBDS, MDent, FC Path (SA)Oral Path. Department of Oral Pathology and Oral Biology, School of Dentistry, Faculty of Health Sciences, University of Pretoria
SUMMARY
The incidence of human papilloma virus-induced oropharyngeal carcinoma is steadily rising globally and the observation has become widely publicised in recent times. Human papilloma virus (HPV) is therefore an important infectious oncogenic agent. The aim of this article is to highlight the modes of transmission in HPV-related oral and oropharyngeal lesions whilst explaining the morphological spectra of benign and malignant disease which are attributed to low-risk and high-risk subtypes respectively. These issues as well as the topic of vaccination against HPV are likely to be the concern of many dental patients. The oral health care worker is therefore expected to provide appropriate counselling and education when informing patients of the potential health risks posed by HPV.
INTRODUCTION
Human papillomavirus (HPV) infection is the most common sexually transmitted viral infection in the world.1 HPV infection is associated with several proliferative, wartlike lesions of the skin and mucosae. High risk HPV-types play a causative role in a significant number of anogenital carcinomas and oropharyngeal carcinomas (OPC's) as well as in carcinomas of sinonasal origin. Due to the common occurrence of benign HPV-induced oral lesions, it is important for the oral health care worker to have a sound knowledge of their clinical manifestations and roles in the health of a patient as well as the current protocol for prevention of infection.
HUMAN PAPILLOMAVIRUS
HPVs are small, double stranded DNA viruses that characteristically infect mucosal and cutaneous epithelium to induce a variety of proliferative lesions.2 More than 170 types of HPV have been identified and are classified as cutaneous or mucosal subtypes on the basis of their preferred site of infection. HPV was initially considered a variant of Polyomavirus but since 2004 has been regarded as a taxonomic family on its own, known as the Papillomaviridae.3 They are divided into five genera on the basis of their genetic composition. Most mucosal HPVs belong to the alpha genus while the beta, gamma, mu and nu genera are mostly associated with skin warts and papillomas.3 The link between HPV and carcinogenesis was first established by zur Hausen in 19774 for which he subsequently received the Nobel Prize in 2008.
Mucosal HPV-subtypes which are strongly associated with cancerous lesions are referred to as "high-risk" and include subtypes 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 70.5 Low-risk mucosal types are present in benign warts and other non-cancerous epithelial lesions and include types 6, 11, 13, 32, 42, 43 and 44. The HPV genome consists of eight open reading frames (ORFs) that are divided into three parts. The early (E) region codes for seven proteins (E1-7) which are needed for viral DNA replication, while the late (L) region codes for two proteins (L1-2) required for viral structure.6 As the most conserved region of the genome, the configuration of the L1 protein is used for identification and classification of the virus.3 A new HPV type is only accepted if the DNA sequence of the L1 ORF differs more than 10% from other types. Differences of between 2-10% define a subtype of HPV and those less than 2% a variant of HPV.3
HPV INFECTION
The clinical features of HPV-associated lesions are dependent on the subtype of HPV as well as the site of infection. HPV infection usually occurs via direct contact and is thus frequently associated with sexual transmission in anogenital lesions. HPV transmission to the oral cavity is mostly through autoinfection in the case of benign lesions such as squa-mous cell papillomas and verruca vulgaris. Sexual activity, especially oral sex, is the most common mode of infection in the case of HPV-related oropharyngeal carcinomas. Direct contact is therefore essential and there is no evidence that HPV is transmitted through saliva on its own.7
HPV infection of epithelium is initiated through the basal cells and the virus most likely gains access following trauma or epithelial erosion when the innate protection afforded by the layers of superficial cells is lost.5 Different receptors facilitating this process have been identified. Binding of HPV to heparin sulphate proteoglycans in the basement membrane appears to be the initial step.8
The tonsillar area is the most prevalent extra-anogenital site for the development of HPV-associated carcinoma, implying early entry of high-risk HPVs into the epithelial lining of the tonsillar crypt. That epithelium is more loosely arranged with a reticulated configuration compared with other epithelial surfaces where the cells are densely packed and joined by desmosomes.9 The tonsillar crypt is therefore well adapted to facilitate contact between ingested antigens and the abundant subjacent lymphoid tissue. The lack of protection afforded by this microscopic arrangement is exploited by the virus for infection of the crypt epithelium.9
Once inside the basal cells, the HPV migrates to the nucleus and remains as an episome (or in a non-integrated state). During normal epithelial maturation the infected daughter cells divide and migrate towards the surface of the epithelial lining. This differentiation triggers the viral genome to initiate the expression of genes required for viral replication, resulting in the shedding of mature viral particles from the epithelial surface.5 The virus does not destroy the infected epithelial cells, as their vitality is required for replication and protection from the immune system of the host.
Cell-mediated immunity is implicated in the control of HPV infection. HPV antigens are however exposed, to a limited extent, to the immune system due to viral proliferation occurring within the epithelial cell.10 Immune evasion is important in the establishment of persistent HPV infection, a prerequisite for neoplastic transformation following infection by high-risk HPV subtypes. If the virus becomes successfully integrated in the genome, a sequence of events follows. Transcription occurs of the E6 and E7 genes which interact with the p53 suppressor gene and retinoblastoma protein (pRb) respectively, resulting in degrading of the p53 protein and inactivation of pRb. This leads to an increase in cell cycling with a reduced capability to repair defective DNA, resulting in cell vulnerability and eventual malignant transformation.
Low-risk HPV subtypes also have E6 and E7 genes and express E6 and E7 proteins. However, the E6 protein in these viral subtypes is unable to degrade p53, while E7 binds with a significantly lower affinity to pRb without the serious complications attributed to the high-risk subtypes.11 The viral genome of low-risk HPVs also remains in a non-integrated episomal form within the nucleus in contrast to the nuclear integration of high-risk subtypes.
ORAL LESIONS
Squamous cell papilloma
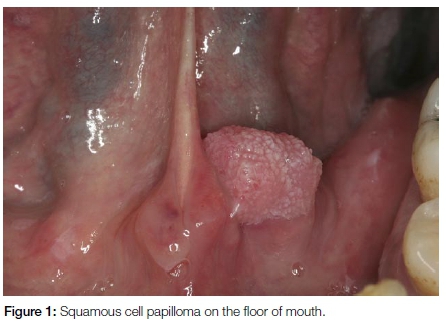
Oral squamous cell papillomas are smaller than 1cm in diameter, painless, can occur anywhere in the oral cavity and involve patients over a wide age range. They have papillary (warty) projections and are often pedunculated (Figure 1). The lesions may have a white appearance if excessive kerat-inisation is present. Squamous cell papillomas are most often associated with HPV-6 and 11 and are not premalignant. Surgical excision is the treatment of choice.
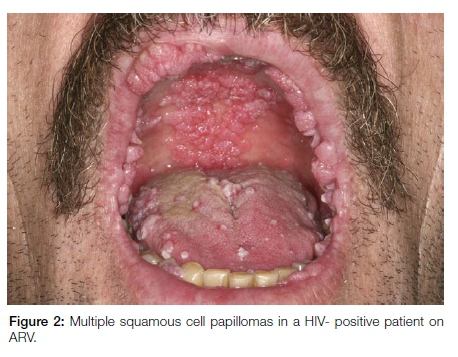
Oral squamous cell papillomas are typically solitary with the exception of lesions noted in HIV/AIDS patients on anti-retroviral therapy. Lesions in this latter group are multiple and multifocal and are frequently larger than the solitary variant (Figure 2). It is postulated that the numerous papillomatous HPV-related benign oral lesions seen in association with antiretroviral therapy (ART) in HIV/AIDS represent a disorder of immune reconstitution.12 ART-associated papillomatous lesions frequently exhibit atypical histological features and may be diagnosed erroneously as HPV-associated dysplastic lesions or as papillary sq-uamous cell carcinoma. However, these well-documented dysplastic features seen in oral papillomas associated with ART are not related to progression to cancer.13 The clinical features are important and should guide the pathologist to a correct interpretation.
Verruca vulgaris
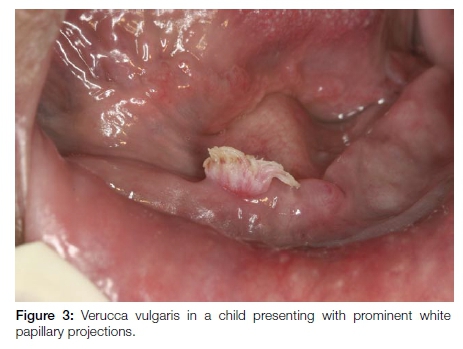
Verruca vulgaris is a common skin wart. Oral lesions are the result of auto-inoculation as reflected by the preferred areas of involvement namely the anterior aspects of the oral cavity, especially the lower lips. The clinical presentation is characteristically that of painless lesions measuring 2-5mm in diameter with pronounced white, papillary projections due to marked hyperkeratosis. (Figure 3) The lesions occur mainly in children. HPV-2, 4 and 57 are typically involved.14 Excision is the treatment of choice although as with the cutaneous warts, oral lesions will often spontaneously resolve, usually within 2-3 years.
Multifocal epithelial hyperplasia
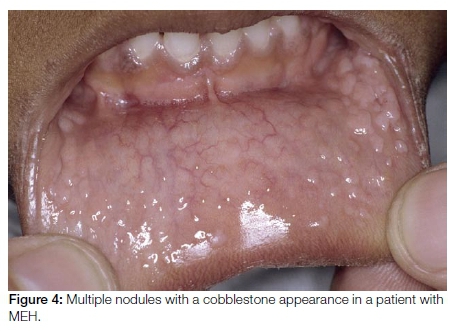
Multifocal epithelial hyperplasia (MEH) also known as focal epithelial hyperplasia or Heck's disease, typically affects children. Many of the affected children are exposed to a crowded environment. MEH presents as multiple mucosal coloured nodules measuring 2-10mm in size with a characteristic cobblestone appearance (Figure 4). The lesions are predominantly present on the lips and gingivae, but can be identified at all oral mucosal sites. HPV types 13 and 32 are the usual causative agents in MEH. Lesions are clinically recognisable and resolve spontaneously within a few-months, obviating the need for treatment. Surgical or medical therapy is only indicated for large lesions which have caused functional and/or severe aesthetic complications.
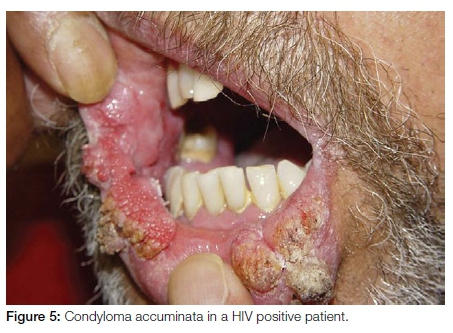
Condyloma accuminatum
Condyloma accuminatum is a sexually transmitted HPV-related squamo-proliferative lesion occurring predominantly in an anogenital location. Oral lesions are transmitted through orogenital sexual contact. The presence of lesions in children should raise the possibility of sexual abuse, although it is possible that HPV may be transmitted by non-sexual contact. This implies that strict criteria should be used when diagnosing oral condyloma accuminata. Condyloma accuminata are larger than squamous cell papillomas and present as multiple broad based, cauliflower-like lesions with blunt processes frequently larger than 1cm (Figure 5). The most common intraoral sites of involvement include the labial mucosa, lingual frenum and soft palate. HPV subtypes 6 or 11 are aetiologically implicated, although high-risk subtypes may also be involved.10 Oral condyloma accuminata can be treated with cryotherapy or surgical excision. If such lesions are clinically or histopathologically diagnosed, it is strongly recommended that the patient undergo testing for the possibility of underlying immune dysfunction, notably for HIV/AIDS.
Oropharyngeal carcinoma
An increase in the incidence of head and neck carcinomas has been observed in the USA recently.15 This is due to an escalation of oropharyngeal carcinoma (OPC) linked to high-risk HPVs, especially HPV-16. The increase is predominantly observed in young males, many of whom had no associated aetiological factors such as tobacco usage or excessive alcohol consumption.16 Similar trends and increases in the incidence of OPC have been reported from different geographical areas worldwide.17
The oropharynx consists of the palatine tonsils, base of tongue and soft palate. Most HPV-associated OPCs develop in the palatine tonsillar area. The tumours have a predominantly endophytic growth pattern and are characterised by early cervical lymph node metastases, which are frequently cystic in nature and may be the only clinical feature at the time of presentation. HPV-associated OPCs have a characteristic non-keratinising histological appearance.
Notwithstanding the advanced clinical stage of these neoplasms at the time of diagnosis, patients with HPV-related OPC have a far better prognosis than those with conventional smoking and alcohol-associated tumours.18 This fact necessitates pathological investigation and detection of a possible HPV-associated aetiology for all tumours occurring at this site. Fortunately, expensive diagnostic techniques are not necessary as immunohistochemical demonstration of p16 protein together with a non-keratinising histologic growth pattern are markers sufficient to confirm HPV involvement in OPC.19 There is good evidence that HPV is of limited importance in squamous cell carcinomas of the oral cavity, regardless of the immunohistochemical expression of p16.20 The vast majority (90-95%) of HPV-associated OPC are attributable to HPV-16. The distinction between oral cavity proper and the oropharynx as sites of cancer is therefore prognostically significant and will have an impact on pathological diagnosis and therapeutic management.
VACCINATION
Due to the infective nature of the HPV associated OPCs, it is reasonable to postulate that vaccination has a potential role in decreasing the spread of the infection with a subsequent drop in the incidence of OPC. Vaccines against HPVs are highly effective in preventing persistent HPV infections and cervical cancer associated with the HPV subtypes covered by the vaccine.21 Three HPV vaccines are currently licenced by the US Food and Drug Administration: a bivalent (HPV-16 and 18) vaccine (Cervarix, GlaxoSmithKline), a quadrivalent (HPV-6, 11, 16 and 18) vaccine (Gardasil, Merck) and a new 9-valent (HPV-6, 11, 16, 18, 31, 33, 45, 52 and 58) vaccine (Gardasil9, Merck).22
HPV vaccination is routinely recommended for adolescents at an age before the onset of sexual activity. The vaccines are used for prevention of the infection and cannot cure an existing HPV infection or an established HPV-associated cancer. Although it makes common sense to vaccinate all boys, the cost prohibits a roll-out of a vaccination program on a national basis. It may be argued that it is not necessary if a successful national vaccination program for girls is in place. This is the case in the UK where the national vaccination program with the quadrivalent vaccine resulted in a 95% coverage in girls. The UK Department of Health therefore only offers vaccination to men who have sex with other men.23
Vaccination for all girls nine years and older in Grade 4 in public schools was implemented in South Africa by the Department of Health in 2014. Ceravix® was awarded the tender and is administered in two doses. The coverage and uptake in public schools is high24 although the uptake in private schools (funded through private health care) is at unacceptably low levels.
South Africa has the highest number of people living with HIV/AIDS and an extremely high incidence of cervical cancer. These worrying statistics suggest that vaccination of boys against high risk HPV types is desirable.25 It has been proven to be a cost effective strategy for the prevention of OPC in several countries.26,27 As mentioned before, the vaccines are expensive and any effective programme will in all likelihood require funding through private health care.
Conflict of interest: None declared
ACRONYMS
ART: antiretroviral therapy
HPV: Human Papilloma Virus
MEH: Multifocal epithelial hyperplasia
OPC: oropharyngeal carcinoma
ORF: open reading frames
References
1. World Health Organization and International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic riks to humans: Human Papillomaviruses. IARC, Lyon, France. 2007; 90: 112-79. [ Links ]
2. zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994;48:427-47. [ Links ]
3. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17-27. [ Links ]
4. zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol 1977;78:1-30. [ Links ]
5. Rautava J, Syrjanen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol 2012;6 Suppl 1:S3-15. [ Links ]
6. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342-50. [ Links ]
7. Prabhu SR, Wilson DF. Human papillomavirus and oral disease - emerging evidence: a review. Aust Dent J 2013;58:2-10; quiz 125. [ Links ]
8. Shafti-Keramat S, Handisurya A, Kriehuber E, et al. Different heparin sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol 2003;77:13125-35. [ Links ]
9. Surjan L, Jr. Immunohistochemical markers of tonsillar crypt epithelium. Acta Otolaryngol Suppl 1988;454:60-3. [ Links ]
10. Pringle GA. The role of human papillomavirus in oral disease. Dent Clin North Am 2014;58:385-99. [ Links ]
11. Ghittoni R, Accardi R, Hasan U, et al. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes 2010;40:1-13. [ Links ]
12. Cameron JE, Hagensee ME. Oral HPV complications in HIV- infected patients. Curr HIV/AIDS Rep 2008;5:126-31. [ Links ]
13. Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:724-31. [ Links ]
14. Castro TP, Bussoloti Filho I. Prevalence of human papillomavirus (HPV) in oral cavity and oropharynx. Braz J Otorhinolaryngol 2006;72:272-82. [ Links ]
15. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer 2005;103:1843-9. [ Links ]
16. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612-9. [ Links ]
17. Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis 2010;16:1671-7. [ Links ]
18. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 2007;121:1813-20. [ Links ]
19. Gondim DD, Haynes W, Wang X, et al. Histologic typing in oropharyngeal squamous cell carcinoma: A four-year prospective practice study with p16 and high-risk HPV mRNA testing correlation. Am J Surg Pathol 2016;40:1117-24. [ Links ]
20. Zafereo ME, Xu L, Dahlstrom KR, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol 2016;56:47-53. [ Links ]
21. Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis 2011;11:13. [ Links ]
22. Centre for Disease Control. HPV vaccine information for clinicians. 2016. Available from: https://www.cdc.gov/hpv/hcp/need-to-know.pdf Accessed: 13 January 2017. [ Links ]
23. Bogaards JA, Wallinga J, Brakenhoff RH, Meijer CJ, Berkhof J. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: Bayesian evidence synthesis. BMJ 2015;350:h2016. [ Links ]
24. Snyman LC, Dreyer G, Botha MH, van der Merwe FH, Becker PJ. The Vaccine and Cervical Cancer Screen (VACCS) project: Linking cervical cancer screening to HPV vaccination in the South-West District of Tshwane, Gauteng, South Africa. S Afr Med J 2015;105:115-20. [ Links ]
25. van Heerden WF, Bunn BK. Oropharyngeal carcinoma: what the dentist should know. SADJ 2012;67:570-2. [ Links ]
26. Graham DM, Isaranuwatchai W, Habbous S, et al. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer 2015;121:1785-92. [ Links ]
27. Wierzbicka M, Jozefiak A, Jackowska J, Szydlowski J, Gozdzicka-Jozefiak A. HPV vaccination in head and neck HPV-related pathologies. Otolaryngol Pol 2014;68:157-73. [ Links ]
 Correspondence:
Correspondence:
Willie FP van Heerden
Department of Oral Pathology and Oral Biology
School of Dentistry, Faculty of Health Sciences, University of Pretoria
Tel: +27 1 2 319 2320
Fax: +27 12 321 2225
E-mail: willie.vanheerden@up.ac.za














