Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.10 Johannesburg Nov. 2016
COMMUNICATION
Oral mucosal ulceration - a clinician's guide to diagnosis and treatment
J FourieI; SC BoyII
IBChD, DipOdont (Perio), DipOdont (Rest), MScOdont (Rest), MChD (Oral Medicine and Periodontics). Department of Periodontics and Oral Medicine. Sefako Makgatho Health Sciences University
IIBChD, MChD (Oral Path), PhD, FCPath(SA) (Oral Path), Department of Oral Pathology, Sefako Makgatho Health Sciences University
ABSTRACT
Oral mucosal ulceration is a common clinical complaint. Ulceration is often debilitating and affects patients from a wide age group. The clinician confronted with such a patient often feels overwhelmed by the different diagnostic possibilities. Given the wide spectrum of conditions encountered, it is striking that the common use of antibiotics and antifungals to treat oral ulceration, is largely inappropriate. This overview provides general dental practitioners (GDP's) and other general healthcare workers with a broad classification of commonly encountered mucosal ulcerative lesions, a practical approach to reach a diagnosis and basic treatment strategies for each condition.
Keywords: Oral, ulceration, vesicle, immune mediated, treatment
Oral mucosal ulceration is a common clinical complaint encountered by medical and oral health care providers. Ulceration involves a breach in the epithelial covering (Figure 1) of mucosa exposing the underlying lamina propria while erosions represent an incomplete breach of the epithelial covering and appears as erythematous patches. Lesions are often debilitating, hampering nutrition and affecting quality of life. The clinician confronted with such a patient often feels overwhelmed, resulting in a kind of shotgun treatment approach that favours the inappropriate use of antibiotics. The aim of this review is to provide the general dentist with a workable classification of more commonly encountered mucosal ulcerative lesions with basic treatment strategies for each.
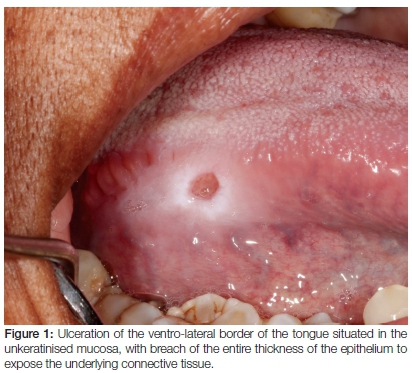
The oral mucosa is biologically subdivided into keratinised (gingiva and palate), non-keratinised (lips, cheeks and ventral tongue) and specialised due to the function of taste (dorsum of the tongue). As a result of its relation to the underlying alveolar bone it may also be divided into masticatory (bone-bound) and non-masticatory. Certain ulcerative conditions affect the keratinised mucosa preferentially whilst others are found almost exclusively on non-keratinised mucosa. The exact location of ulcers is therefore crucial information to assist with the correct diagnosis.
Classification of oral mucosal ulceration may be according to the (i) clinical features, (ii) microscopic features or (iii) pathogenesis (traumatic, metabolic, dermatological, allergic, immunological, infectious, and neoplastic) but classification according to (iv) the clinical presence or absence of preceding fluid-filled vesicles/ bullae will form the basis for the discussion in this review (Figure 2). Secondary information such as systemic signs and symptoms, location, duration and number (single or multiple) of ulcerations are supplied for each condition to guide the clinician to a more focused differential diagnosis. Vesicles (depending on the size are also termed bleb, bulla or blister) are the clinical manifestation of separation of either epithelial cell layers from each other or from the underlying lamina propria that subsequently become filled with fluid. The final diagnosis of oral mucosal ulcerations is usually dependent on performing a biopsy and submitting the tissue to a pathologist for histological diagnosis. Special investigations in the form of stains and immunofluorescence are often necessary and the pathologist will employ these as necessary.
Topical corticosteroids in the management of oral ulcerative conditions
The anti-inflammatory and immunosuppressive properties of corticosteroids render them ideal for the management of especially immune mediated oral ulcerative conditions. Side effects of systemic corticosteroids make them less favourable as primary treatment options and topical steroids are often good alternatives. Kenalog in Orabase® (triamcinolone acetonide 0.1%) was often the agent of choice but since its discontinuation alternatives have to be considered (Table 1).
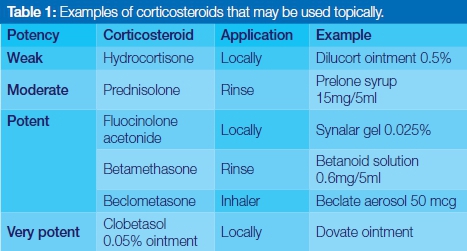
The potency of the topical steroid, frequency and vehicle of application should be tailored to each individual case and response to treatment. Generally, widely distributed ulceration may be more effectively treated with a corticosteroid rinse, single lesions with the local application of an ointment, and oropharyngeal lesions with a steroid inhaler. Ointments are not adhesive to the oral mucosa but may be combined with either orabase1 or denture adhesives.2 Topical corticosteroids are considered in the following clinical settings: (i) as short course to speed up recovery e.g. recurrent aphtous stomatitis (RAS), erythema multiforme and drug induced ulceration; (ii) sporadic use in conditions with chronic, often cyclical clinical course i.e. oral lichen planus (OLP), severe RAS and mucous membrane pemphigoid (MMP); (iii) maintenance therapy following a short course of systemic corticosteroids i.e. severe OLP; (iv) concurrently with systemic immunosuppressive therapy to reduce the dosages thereof i.e. pemphigus vulgaris.3
ULCERS PRECEDED BY VESICLES (VESICULO-ULCERATIVE LESIONS)
Patients may present with or without intact vesicles preceding the ulcers. Vesicles are often lost due to functional oral activity, remaining only in protected areas of the mouth or in diseases with thick-roofed sub-epithelial vesicles i.e. MMP. Irrespective, patients frequently provide a history of "blisters" preceding the ulcerations or previous episodes of ulcerations and that, together with the specific mucosal region affected provide mandatory information to formulate a correct diagnosis. Generally, lesions in this category that presents with an acute onset are likely of viral origin, while lesions with a chronic or relapsing course more likely immune mediated.
I Infections: Vesiculo-ulcerative lesions of viral origin
Preceding systemic signs and symptoms are typically present.
Herpes Simplex Virus (HSV)
The most common to affect the oral mucosa, primary HSV-1 infection mostly affects children presenting either as asymptomatic infection or with mucosal vesicles followed by painful ulceration affecting both keratinised and non-keratinised mucosa. Adults with primary infection suffer symptomatic herpetic pharyngotonsillitis initiated as vesicles that rapidly break down into painful shallow ulcerations. Serological investigations at this stage demonstrate absence of HSV-1 immunity. Primary infection normally runs a self-limiting course but initiation of acyclovir suspension within 72 hours of onset (15mg/kg, 5 times/day for 7 days) reduces symptoms with shorter duration of the ulcers and decreased viral shedding.4,5 The later in life primary infection occurs, the greater the need for antiviral therapy, with 1g valacyclovir given twice daily for adults.5 Recurrent infections follow the dermatome of the ganglion in which the virus established latency after the primary infection.6 Recurrences in the form of herpes labialis are most commonly initiated by various factors including, but not limited to, stress, UV exposure or dental local anaesthetic. Initial prodromal stinging or burning is followed by a cluster of approximately five small fluid-filled vesicles on erythematous mucosa that ruptures to leave painful shallow ulcers which coalesce and crusts. Despite low bioavailability of the active metabolite and multiple applications needed, herpes labialis is effectively treated by 5% acyclovir or 1% pencyclovir cream when initiated within the prodromal stages.4 Systemic antiviral agents with significant anti-HSV action (acyclovir, valacyclovir, famcyclovir) may be used early in recurrence,4,7 although results are conflicting.8 Addition of corticosteroids to the topical formulation of antiviral agents significantly decreases the ulcerations but adds no benefit to the resolution time.9
Herpangina (Hand-Foot-Mouth Disease)
Caused by coxsackievirus, echoviruses, and other enteroviruses, typically affects children below 10 years. Two to six red macules or vesicles is followed by self-limiting ulcerations, approximately 5mm in diameter, on the anterior tonsillar pillars, soft palate, uvula, and/ or tonsils. Pyrexia, sore throat and headaches are common. Ulcers heal within 4-6 days and management is symptomatic with analgesics and antipyretics. Anti-inflammatory (benzydamine) and antimicrobial (chlorhexidine) formulations such as Andolex C® may be used but not antivirals.10
Varicella Zoster (HHV-3)
Varicella is well-known for its pruritic, vesicular skin rash, ulceration and crusting, all occurring concurrently.11Crusting is absent in the oral mucosa which instead present as ulcerating papules. A mouthwash compounded from equal parts viscous lidocaine, diphenhydramine (Benadryl®) and Maalox has been advocated although acyclovir 80mg/kg/day divided in four doses for children and 4g/day divided into 5 doses for adults for 5 days instituted within 24 hours of onset of the rash may reduce duration of the lesions.11 Herpes zoster (shingles) signifies recurrent HHV-3 infection, mostly affecting old and debilitated patients, and follows the dermatome of the ganglion in which the virus established latency. Severe burning or stinging pain to the affected dermatome is followed by fluid filled vesicles that rupture to leave painful shallow ulcerations that may coalesce to form large denuded areas. Oral manifestations signify involvement of the mandibular or maxillary divisions of the trigeminal nerve with pathognomonic abrupt termination of lesions along the midline. Osteonecrosis with tooth exfoliation has been reported, especially in immune deficient individuals.12,13 Post-herpetic neuralgia is defined as persistence or recurrence of pain more than a month after onset of shingles and is seen with increased frequency in old patients with facial infection. Administration of antiviral drugs within 72 hours of onset of the rash14 is recommended in the elderly, immune deficient patients and those with facial shingles.14 Oral acyclovir (800mg, 5 times/day for 7 to 10 days), valacyclovir 1000mg (3 times/day for 7 days) and famcyclovir 500mg (3 times/day for 7 days) are treatments of choice but does not reduce the incidence of post-herpetic neuralgia.15 Corticosteroids (i.e. 0.5mg prednisone/kg/day) may relieve the acute phase zoster-associated pain.16,17 The Advisory Committee on Immunization Practices (ACIP) of the Centre for Disease Control and Prevention (CDC) recommend the routine use of herpes zoster vaccine in patients over 60 years18 (Zostavax®).
II Immune-mediated vesiculo-ulcerative lesions
Several dermatological conditions may present with oral mucosal involvement, either concurrent with the skin pathology, as the initial presentation or sometimes as the only clinical presentation.19 Ulcerations have a tendency to be chronic and the systemic signs and symptoms associated with infection usually absent. All immune-mediated forms of mucosal ulceration have to be diagnosed via microscopic examination of a surgical biopsy which preferably contains the transitional zone between the ulcerated and adjacent normal appearing mucosa. The demonstration of tissue-bound or circulatory antibodies via direct (DIF) or indirect immunofluorescence (IIF) techniques on peri-lesional mucosal biopsy is diagnostic.
Pemphigus vulgaris (PV)
Pemphigus, a group of immune mediated mucocutaneous diseases, is mediated by auto-antibodies directed at the proteins of keratinocyte adhesion (desmosomes) causing acantholysis. PV most commonly affects the oral cavity, it's autoantibodies mainly directed against desmoglein 1 and 3 (mucocutaneous forms) or only 3 (mucosal forms).20 Patients, typically 40-60 years of age, present with thin-roofed, flaccid intra-epithelial bullae which rupture promptly after development resulting in large irregular areas of painful mucosal ulceration. Pressure applied laterally on a bulla or healthy appearing skin/mucosa, may cause sloughing of the adjacent epithelium, a phenomenon known as positive Nikolsky sign. Oral lesions, seen mostly in areas of friction, are often the initial and sometimes the only presentation of the disease. Microscopic evaluation confirms intra-epithelial vesicle formation with inter-epithelial deposition of mainly IgG antibodies on DIF. The aim of treatment is to bring the disease under control, mostly using systemic corticosteroids, followed by a maintenance period of the minimum dose corticosteroid required for disease control.21 Treatment withdrawal with complete and durable remission is obtainable in roughly 75% of patients after 10 years.22 Systemic corticosteroids are the best management option20 although the optimal dosing schedule varies. Mild cases are initiated on 40-60mg prednisone/day and more severe cases on 60-100mg/day as evidenced by the degree of skin surface involved and the rate of appearance of new vesicles. If no improvement is evident after 5-7 days, dosages should be increased by increments of 50% per week. Once remission is achieved and most lesions have healed, doses can be tapered down to the lowest disease controlling levels. Azathioprine at 1-3mg/kg/day titrated against thiopurine methyl transferase (TPMT) levels is the adjuvant drug of choice and should be instituted simultaneously with the systemic corticosteroids. Topical corticosteroids (rinse or ointment) may improve oral lesions and may be considered as monotherapy in mild disease where only the oral mucosal surfaces are affected.23
Mucous membrane pemphigoid (MMP)
A common systemic autoimmune blistering disease with preferential involvement of mucosal membranes. The antibodies are directed at the proteins of keratinocyte to connective tissue matrix adhesion or hemi-desmosomes (BP180 and laminin-332)20 causing the epithelium to split away from its underlying connective tissue bed. The sub-epithelial nature of the split results in thick roofed vesicles which may still be intact on examination. Rupture of the vesicles leave ulcerative lesions devoid of any epithelium, covered by yellow-white slough. Desquamative gingivitis (erythematous and friable gingiva with epithelial destruction) is a frequent finding.24 Microscopy confirms sub-epithelial vesicle formation with linear deposition of IgG and/ or IgA antibodies and compliment in the basal membrane zone on DIF. Management difficulties are ascribed to diverse pathogenic pathways, lack of well-designed clinical trials, variability in disease severity and different efficacies of treatments on different mucosal surfaces.25,26 Patients with oral mucosal involvement only or with limited additional skin involvement, can be managed with moderate-to high potency topical corticosteroids, dapsone (50-200mg/day) or tetracycline (1-2mg/day) with added nicotinamide (2-2,5mg/day). Patients with diffuse oral mucosal and extra-oral mucosal surface involvement are managed with systemic corticosteroids (prednisone 0,5mg - 1,5mg/kg/day). Alternatively, corticosteroid sparing agents such as azathioprine, or mycophenolate mofetil may be supplemented by topical corticosteroids on affected mucosal sites.25,26
Erythema multiforme (EM)
Erythema multiforme (EM) is a T-cell-mediated type IV cytotoxic immune reaction to a variety of antigens (viral, bacterial, pharmacological, or chemical) that result in apoptosis-mediated epithelial cell death.19 Anti-desmoplakin I and II antibodies were recently demonstrated as a possible instigator of the cytotoxic reaction.27 Previously considered to be a spectrum of clinical conditions, EM, formerly known as EM minor, is now accepted as a distinct entity.19 EM mostly affects young, healthy individuals and is often recurrent and temporal with recurrent HSV infections.28 Target lesions of skin are pathognomonic round, oedematous, erythematous papules with well-defined border and paler central vesicle. Oral lesions may either represent the start of further mucocutaneous involvement or may appear in isolation, classically with swollen, cracked, haemorrhagic and crusted lips with or without mucosa blisters and ulcerations.29 Treatment is targeted at the initiating organism. Patients with herpes associated EM often reports a history of recent recurrent HSV infection while the less common drug induced EM is not recurrent. Topical corticosteroids may be sufficient for EM supplemented with disinfecting and anesthetising mouthrinses (Andolex-C®).30 EM associated with HSV can be treated with a 5 day course of acyclovir (5 times/day) which should be instituted at the first sign of lesions.29 Prophylaxis in the form of twice daily administration of acyclovir (400mg), valacyclovir (500mg) or famcyclovir (250mg) for 6 months may be considered in long term management of frequent recurrences. In patients unresponsive to long term viral suppression, azathioprine, dapsone, mycophenolate mofetil, chloroquine, thalidomide or cyclosporine may be of benefit to suppress the aberrant immune response.30 Mycoplasma associated EM is treated with tetracycline.29
ULCERS NOT PRECEDED BY VESICLES
This group of oral mucosal ulceration represents the larger of the two groups and includes a wide differential diagnosis which should be considered against a proper clinical history and specific clinical features.
I Mucosal Infections
Infectious mucosal ulceration not preceded by blisters should generally be considered to be of bacterial or fungal nature rather than viral.
Common bacterial infections
Syphilis
Treponema pallidum infection continues to be widespread, with increasing rates among men who have sex with men.31 The primary lesion presents at the first site of mucosal inoculation, frequently the oral mucosa. A highly infective, painless, solitary ulcer with indurated margins and ipsilateral lymphadenopathy is the most common, with healing within three weeks. Non-characteristic mucous patches alerts to the development of secondary syphilis frequently accompanied by a maculo-papular rash of the palmo-plantar surfaces of the hands and feet, and generalised lymphadenopathy.32 Primary, secondary and early latent syphilis (less than one year since primary infection) require a single dose of parenteral Benzathine penicillin G.33
Tuberculosis (TB)
The emergence of drug-resistant TB and the high numbers of HIV-infected individuals in South Africa has resulted in an increase of TB cases urging inclusion in the differential diagnoses of orofacial pathology. Secondary TB in the form of painful, deep irregular ulcers with indurated appearance, undermined edges and thick mucus-like material at the base of any aspect of the tongue are typical. Haematogenous spread from pulmonary TB or secondary inoculation of a traumatic ulcer with infected sputum is the most common pathogenesis. Primary oral TB is distinctly rare, usually associated with Mycobacterium bovis. Ulcers resemble chronic traumatic ulceration and even malignancy urging a diagnostic biopsy.34,35 Associated symptoms of pain, fever, lymphadenopathy, hoarseness of voice and weight loss frequently accompany the ulcerations. The diagnosis is confirmed by a biopsy, special stains to visualise the acid fast bacilli or traditional culture methods for positive identification of the organisms. Molecular diagnostics of TB is available but high cost and decreased accuracy in the HIV-positive population is problematic in the South African setting.36
Necrotising ulcerative gingivitis (NUG) / periodontitis (NUP)/ stomatitis (NUS)
An opportunistic gingival infection caused by an array of bacteria in malnourished children, young adults and immune deficient patients. NUG is often the initial presentation, proceeding into NUP, NUS and ultimately noma. Necrosis and ulceration of the interdental gingival papilla, exquisite pain, severe halitosis, regional lymphadenopathy, malaise and fever differentiate this form of ulceration from others. When the alveolar bone becomes exposed, necrotic bone sequestrae may develop and should be removed with the associated teeth.37 Treatment includes pain control through analgesics, antimicrobials, and antiseptic mouthrinses containing chlorhexidine (Andolex C®).38 Metronidazole (400mg 3 times/day) is given to target the anaerobic organisms,39 while a broad spectrum antibiotic (amoxicillin 500mg 3 times/day) may be added in severe cases. Professional mechanical debridement (scaling and polishing) and institution of proper plaque control is essential. Predisposing factors, such as cigarette smoking and immune suppression, should be investigated and eliminated where possible. Morphological defects of the gingival architecture can be surgically corrected and the patient closely maintained to prevent recurrence.38
Common fungal infections: Aspergillus and Mucormycosis
Both superficial and invasive opportunistic fungal infections are encountered in the oral cavity of especially immunocompromised patients. Candida infection is unlikely to present as or cause oral ulceration and should not be included in a differential diagnosis for oral ulceration. Mycoses to be considered include zygomycosis, aspergillosis, histoplasmosis, blastomycosis, and paracoccidioidomycosis. Aspergillus and Mucomycosis, albeit uncommon, are the most commonly encountered and follows the inhalation of the spores from soil, manure, grain, cereal and mouldy flour.40,41 Generally both organisms have a propensity to penetrate the walls of small to medium-sized blood vessels, resulting in thrombosis, infarction, tissue necrosis and ulceration. Necrosis results in the exposure of necrotic bone, loss of adjacent teeth and in some instances palatal perforation. Presence of the organisms is confirmed by tissue biopsy with special stains to visualise the fungal hyphae. Histomorphological features may assist in fungus recognition but culture is needed for definitive confirmation.42 Treatment of aspergillosis depends on whether it is invasive or not (mycetoma and allergic fungal sinusitis respectively). Invasive aspergillosis and mucormycosis is treated by radical surgical debridement and intravenous Amphotericin B or the liposomal variant (1-1.5mg/kg/day).40-43 Itraconazole, voriconazole and intravenous caspofungin may also be considered.43
II Immune-mediated diseases not preceded by vesicles
Recurrent aphthous stomatitis (RAS)
RAS represents the most common form of oral mucosal ulceration encountered in healthy individuals.44,45 The term should be reserved for recurrent ulcers of the oral mucosa, not associated with any systemic disease and which typically commence in childhood or adolescence. Non-keratinised mucosa of the buccal mucosa, lips and soft palate is most commonly affected in contradiction with recurrent HSV seen on keratinised mucosa of the vermillion, gingiva and hard palate. A variety of local and systemic factors including immunologic, allergic, nutritional, microbial organisms, psychosocial stress as well as immunosuppressive drugs, have been proposed as possible etiological factors.44 Increased prevalence in close family members also indicate a possible genetic background.46 RAS has an atypical clinical presentation in HIV-infected patients and should always be considered as differential diagnosis of oral mucosal ulceration in them.47 When RAS starts later in life, additional mucosal surfaces may be affected and a comprehensive physical examination and medical history should be considered to rule out inflammatory gastrointestinal disease such as Crohn's, Coeliac disease, Behçet's syndrome, Sweet's syndrome, cyclic neutropenia, HIV infection and drug reactions in which case "aphthous-like ulcers" is a more appropriate term.45 Clinically RAS is classified according to the size of the ulcers, number, location and healing period of the lesions. Minor RAS is the most common variant and the patient typically present with 1-5 ulcers, less than 10mm in diameter surrounded by a bright red inflammatory halo. Ulcers heal spontaneously within 10-14 days. RAS major (Sutton disease) usually appears after puberty and present as deeper, larger, persistent ulcerations with more irregular borders than minor RAS. One to 10 ulcers, usually >10mm in diameter typically take weeks or months to heal. Systemic symptoms such as fever and malaise may accompany the severe dysphagia associated with these lesions which tend to occur in the posterior palate and pharynx and heal with scarring. Herpetiform type RAS are clinically distinct and bears no relationship to HSV infection. It presents mostly commonly in females aged 20-29 years as clusters of 10-100 pinpoint ulcers on the lateral border of the tongue which may coalesce to form large painful ulcerations. RAS is usually preceded by a prodrome of burning pain 1 to 2 days before the extremely painful ulcers covered by pseudomembranes and surrounded by a characteristic flame red halo appears. Treatment strategies depend on the type of RAS in a particular patient but relies mostly on eliminating any instigating or aggravating factors, be it mechanical trauma (toothbrush, food or orthodontic brackets), chemical irritation (some patients relate the onset of ulcers to particular foods) or microbiological (maintaining optimal oral hygiene). Topical analgesics such as 2% lidocaine hydrochloride or benzydamine (Andolex®) may be considered to numb the mucosal pain. The disease process itself can be manipulated by using topical corticosteroids in rinses, ointments or aerosols depending on the distribution and location of the lesions with ointments favoured for solitary lesions that the patient can reach and steroid inhalers used for lesions located on the soft palate or oropharynx. Alternatively a tetracycline mouth rinse (such as a 100mg doxycycline capsule dissolved in 25ml of water and rinsed with 3 times/day) may be used due to its anti-inflammatory properties. Systemic immune modulating agents such as glucocorticosteroids, dapsone, thalidomide and azathioprine should be reserved for more severe cases.48
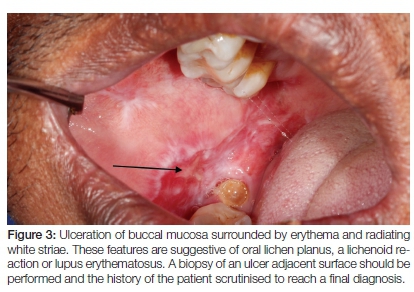
Oral lichen planus (OLP)
OLP is a rather common, chronic inflammatory disorder affecting mainly middle-aged females. The pathogenesis remains uncertain but various subsets of T-lymphocytes and mast cells play a role in the basal membrane damage.49 The disease may present with a diverse clinical spectrum which includes the atrophic, erosive, ulcerative and less commonly, bullous variants.50 A mixture of clinical variants with or without desquamative gingivitis is possible. The lesions typically affect the oral mucosa bilaterally and are fairly symmetric, presenting as either solely an oral mucosal disease or be accompanied by cutaneous manifestations. In the case of the erosive/ ulcerative types, painful pseudomembrane covered ulcerations bordered by faint white striae are seen in a multifocal distribution. The white striae should always alert the clinician to the possibility of OLP (Figure 3). Malignant transformation of OLP remains a contentious issue. Recent meta analyses determined the overall malignant transformation rate to be around 1.09%, most commonly affecting the tongue of older females.51,52 Continuous evaluation and follow-up remains important. Treatment is aimed at relief of symptomatic atrophic and ulcerative lesions only. All aggravating factors such as mechanical trauma from sharp teeth and restorations and chemical irritation from foods and dentifrices should be eliminated.53 Corticosteroids, and especially topical application thereof, have been most extensively investigated. The potency of the steroid and the formulation used (rinse vs. ointment) should match the severity and distribution of the oral lesions. Systemic steroid use is limited to cases with severe or widespread ulceration, treatment resistance and involvement of multiple sites.54 Long-term use of topical corticosteroids is associated with a higher incidence of Candida infection.55 Intra-lesional corticosteroids can be used to target ulcerative lesions (Depo-Medrol 40mg/ml with lidocaine 10mg/ml).56 Only in symptomatic OLP or those with extra-oral involvement resistant to conventional therapy should steroid sparing agents such as chloroquine or azathioprine be considered.54
Allergic reactions
Oral mucosal hypersensitivity reactions are less common than cutaneous ones ascribed to the possible allergen dilution and the continuous rinsing effects of normal saliva flow.57 Lesions may imitate lichen planus or present with non-specific tissue oedema, erythema, cracking, ulceration, hyperkeratotic white plaques or mucosal desquamation.58 Lesions may start long after the introduction of a drug and may remain for months after cessation thereof59 complicating diagnosis and management. Three forms of allergic mucosal reactions are discussed briefly.
Lichenoid lesions
When a hypersensitivity reaction to either a systemic drug or direct contact with an offending agent results in clinical and histological features reminiscent of lichen planus, the term 'oral lichenoid drug reaction (OLDR)' or 'oral lichenoid contact lesion'(OLCL)' is used respectively. A temporal or spatial association with an offending agent can usually be identified.54 Amalgam is often implicated in OLCL, confirmed by patch testing for mercury or amalgam sensitivity.60 It would however be reasonable to replace a suspicious restoration when patch testing is not available. OLDR is encountered with some frequency in patients treated with angtiotensin-converting enzyme (ACE) inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs) and oral hypoglycaemic drugs.54 Regardless of the eliciting allergen, both OLCL and OLDR may present with significant ulceration, usually with remarkable erythema and white striations at the periphery of the ulceration reminiscent of LP.
Fixed drug eruption (FDE)
This form of hypersensitivity is remarkable for its fixed anatomical nature and has been described with NSAID's and other oxicam drugs,61 gabapentin,62 fluconazole,63 systemic antibacterial and antifungal drugs.64 FDE should be suspected in cases with a temporal association of drug ingestion, may be confirmed through patch testing or oral provocation tests, and managed through drug avoidance or substitution, while the acute lesions can be treated with topical or systemic steroids.
Allergic contact stomatitis
Although rare, this form of mucositis have been reported in association with dental impression materials,65 dental restorative materials,57 topical benzocaine application66 and more commonly cinnamon in toothpastes, mouth rinses, and chewing gum.67 Lesions may appear as mixed red and white patches with ulceration, features that may resemble leukoedema, lichen planus, lupus, leukoplakia and verrucous white plaques, swelling of the cheeks and gingival desquamation. The lesions may appear on the lips, cheeks, tongue and gingiva as localised or widely distributed lesions.68
Lupus erythematosus (SLE/DLE)
More than half of patients with systemic lupus erythematosus (SLE) may present with oral lesions, most frequently ulceration of the buccal mucosa and lips during the early, active disease phase.69 Ulcerative lesions and erythematous lesions with or without radiating white striae may also be seen as part of the clinical spectrum of discoid LE (DLE).70 DLE is considered a potentially malignant disorder of the oral mucosa due to the increased prevalence of OSCC among this population, especially involving the lower lip.71 Both physical and chemical sun protective strategies should therefore be implemented. Treatment of patients with oral lesions of SLE/ DLE should be targeted at the systemic/ dermatologic condition which includes the use of nonsteroidal anti-inflammatory drugs, antimalarial agents, glucocorticoids and immune suppressive drugs such as cyclophosphamide, azathioprine, methotrexate and mycophenolate mofetil.72 Literature regarding the specific treatment of the oral lesions is scarce but topical use of corticosteroids of varying potency is accepted as the general standard of care.70 The chosen formulation will depend on the distribution and accessibility of the lesions.
III Trauma
Traumatic ulceration of the oral mucosa may be acute or chronic in nature with the latter diagnostically more problematic due to underlying fibrosis and clinical appearance of neoplastic induration. A thorough clinical history will often alert the clinician to a traumatic aetiology or burns caused by warm food or chemicals whilst the intra-oral examination may reveal the causative factor such as sharp broken tooth or restoration or ill-fitting denture. Ulceration due to local anaesthetic most often occurs in the hard palate, the combined result of pressure and ischemic necrosis. A special kind of chronic traumatic ulceration (Riga Fede disease) is sometimes observed on the ventral surface of the tongue of infants with natal, neonatal or even early erupted primary teeth.73 These ulcerations may interfere with feeding and treatment is essential. If the tooth is a supernumerary tooth, it may be extracted. If these teeth are however the only primary teeth, a soft formed mouth guard for feeding74 or covering the sharp incisal edges with restorative material73 may be performed to protect the opposing soft tissue. Any persistent ulcer should be biopsied to rule out malignancy.
IV Neoplasia
Primary and metastatic malignancies
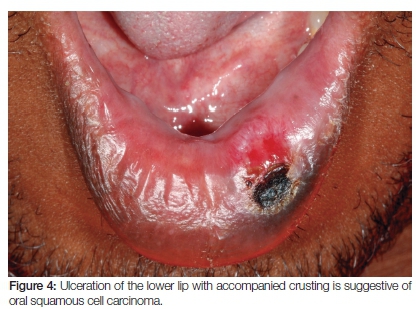
The oral mucosa may be affected by an array of both primary and metastatic malignancies which may all present as non-specific ulcers. Oral squamous cell carcinoma (OSCC) is the most common, frequently presenting as ulceration with clinical induration, fixation to the underlying tissues, rolled exophytic margins, pain and/or numbness (Figure 4). Histopathological examination of all ulcerated, red, white or mixed oral lesions that have persisted for more than three weeks is mandatory. Metastatic malignancy to the jaws heralds a poor prognosis, often clinically resembling reactive or traumatic lesions, periodontal conditions and even inflammatory enlargement.75
Antineoplastic therapy induced mucositis
Previously thought to be the result of direct epithelial damage caused by cytotoxic therapy, is now known to involve a complex cascade of events which is initiated by reactive oxygen species76 with extensive inflammation, atrophy, swelling, erythema and ulceration.77 Chemotherapy-induced mucositis starts within 4-7 days of initiation and peaks within two weeks. Healing occurs only after cessation of therapy. Radiation induced mucositis may be seen following a cumulative dose of 15 Gy and tends to reach full severity at 30 Gy which may last for months.77,78 Radiation lesions correspond to the exposed surfaces while chemotherapy induced mucositis affects the entire alimentary tract. The type and dosage of systemic cytotoxic agents, and the dosage and field of radiation will affect the presence and severity of mucositis. Evidence based guidelines for the management of cancer therapy induced oral mucositis was established and should be referred to in all cases of patients receiving these agents.77 Dental examinations and treatment are however recommended for all patients receiving oncotherapy, especially patients with head and neck cancers.
CONCLUSION
Exploration of the different conditions responsible for oral ulceration reveals marked distinction between lesions that are preceded or accompanied by vesicles and those that are not. Accurate and complete history taking and consideration of the anatomical location of the lesion is therefore essential in reaching an accurate working diagnosis which can be successfully managed with the recommendations provided.
Acknowledgements
Dr Aubrey Masilana for supplying the clinical case photographs.
No financial support was received, and there is no conflict of interest in the publication of this work.
ACRONYMS
ACE: angtiotensin-converting enzyme inhibitors
EM: Erythema Multiforme
HS: Herpes Simplex Virus
SLE/DLE: Lupus Erythematosus
MMP: Mucous Membrane Pemphigoid
NUG: Necrotising Ulcerative Gingivitis
NSAIDs: Nonsteroidal Anti-Inflammatory Drugs
NUG: Necrotising Ulcerative Gingivitis
OLP: Oral Lichen Planus
OLCL: Oral Lichenoid Contact Lesion
OLDR: Oral Lichenoid Drug Reaction
OSCC: Oral squamous cell carcinoma
PV: Pemphigus Vulgaris
TB: Tuberculosis
RAS: Recurrent Apthous Stomatitis
HHV-3: Varicella Zoster Virus
References
1. Lozada-Nur F, Miranda C. Oral lichen planus: topical and systemic therapy. Semin Cutan Med Surg. 1997;16(4):295-300. [ Links ]
2. Lo Muzio L, della Valle A, Mignogna MD, Pannone G, Bucci P, Bucci E, et al. The treatment of oral aphthous ulceration or erosive lichen planus with topical clobetasol propionate in three preparations: a clinical and pilot study on 54 patients. J Oral Pathol Med. 2001;30(10):611-7. [ Links ]
3. Gonzalez-Moles MA, Scully C. Vesiculo-erosive oral mucosal disease--management with topical corticosteroids: (1) Fundamental principles and specific agents available. J Dent Res. 2005;84(4):294-301. [ Links ]
4. Arduino PG, Porter SR. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: review of its management. Oral Dis. 2006;12(3):254-70. [ Links ]
5. Amir J, Harel L, Smetana Z, Varsano I. Treatment of herpes simplex gingivostomatitis with aciclovir in children: a randomised double blind placebo controlled study. BMJ. 1997;314(7097):1800-3. [ Links ]
6. Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A Comparison of HSV-1 and VZV Latency and Reactivation. J Gen Virol. 2015. [ Links ]
7. Spruance SL, Bodsworth N, Resnick H, Conant M, Oeuvray C, Gao J, et al. Single-dose, patient-initiated famciclovir: a randomized, double-blind, placebo-controlled trial for episodic treatment of herpes labialis. J Am Acad Dermatol. 2006;55(1):47-53. [ Links ]
8. Rahimi H, Mara T, Costella J, Speechley M, Bohay R. Effectiveness of antiviral agents for the prevention of recurrent herpes labialis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(5):618-27. [ Links ]
9. Arain N, Paravastu SC, Arain MA. Effectiveness of topical corticosteroids in addition to antiviral therapy in the management of recurrent herpes labialis: a systematic review and meta-analysis. BMC Infect Dis. 2015;15:82. [ Links ]
10. Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341(13):936-42. [ Links ]
11. Heininger U, Seward JF. Varicella. Lancet. 2006; 368(9544): 1365-76. [ Links ]
12. Mendieta C, Miranda J, Brunet LI, Gargallo J, Berini L. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontol. 2005;76(1):148-53. [ Links ]
13. van Heerden WF, McEachen SE, Boy SC. Alveolar bone necrosis and tooth exfoliation secondary to herpes zoster in the setting of HIV/AIDS. AIDS. 2005;19(18):2183-4. [ Links ]
14. Whitley RJ, Volpi A, McKendrick M, Wijck A, Oaklander AL. Management of herpes zoster and post-herpetic neuralgia now and in the future. J Clin Virol. 2010;48 Suppl 1:S20-8. [ Links ]
15. Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866. [ Links ]
16. Whitley RJ, Weiss H, Gnann JW, Jr., Tyring S, Mertz GJ, Pappas PG, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125(5):376-83. [ Links ]
17. Han Y, Zhang J, Chen N, He L, Zhou M, Zhu C. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;3:CD005582. [ Links ]
18. Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR, Centers for Disease C, Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-31. [ Links ]
19. Bascones-Martinez A, Garcia-Garcia V, Meurman JH, Requena-Caballero L. Immune-mediated diseases: what can be found in the oral cavity? Int J Dermatol. 2015;54(3):258-70. [ Links ]
20. Ishii K. Importance of serological tests in diagnosis of autoimmune blistering diseases. J Dermatol. 2015;42(1):3-10. [ Links ]
21. Bystryn JC, Rudolph JL. Pemphigus. Lancet. 2005; 366(9479): 61-73. [ Links ]
22. Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42(3):422-7. [ Links ]
23. Harman KE, Albert S, Black MM, British Association of D. Guidelines for the management of pemphigus vulgaris. Br J Dermatol. 2003;149(5):926-37. [ Links ]
24. Hasan S. Desquamative gingivitis - A clinical sign in mucous membrane pemphigoid: Report of a case and review of literature. J Pharm Bioallied Sci. 2014;6(2):122-6. [ Links ]
25. Taylor J, McMillan R, Shephard M, Setterfield J, Ahmed R, Carrozzo M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(2):161-71 e20. [ Links ]
26. Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370-9. [ Links ]
27. Fukiwake N, Moroi Y, Urabe K, Ishii N, Hashimoto T, Furue M. Detection of autoantibodies to desmoplakin in a patient with oral erythema multiforme. Eur J Dermatol. 2007;17(3):238-41. [ Links ]
28. Carrozzo M, Togliatto M, Gandolfo S. [Erythema multiforme. A heterogeneous pathologic phenotype]. Minerva Stomatol. 1999;48(5):217-26. [ Links ]
29. Farthing P, Bagan JV, Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 2005;11(5):261-7. [ Links ]
30. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51(8):889-902. [ Links ]
31. Stoltey JE, Cohen SE. Syphilis transmission: a review of the current evidence. Sex Health. 2015. [ Links ]
32. Hertel M, Matter D, Schmidt-Westhausen AM, Bornstein MM. Oral syphilis: a series of 5 cases. J Oral Maxillofac Surg. 2014;72(2):338-45. [ Links ]
33. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312(18):1905-17. [ Links ]
34. Jain P, Jain I. Oral Manifestations of Tuberculosis: Step towards Early Diagnosis. J Clin Diagn Res. 2014;8(12):ZE18-21. [ Links ]
35. Von Arx DP, Husain A. Oral tuberculosis. Br Dent J. 2001;190(8):420-2. [ Links ]
36. Vittor AY, Garland JM, Gilman RH. Molecular Diagnosis of TB in the HIV Positive Population. Ann Glob Health. 2014;80(6):476-85. [ Links ]
37. Feller L, Altini M, Chandran R, Khammissa RA, Masipa JN, Mohamed A, et al. Noma (cancrum oris) in the South African context. J Oral Pathol Med. 2014;43(1):1-6. [ Links ]
38. Bermejo-Fenoll A, Sanchez-Perez A. Necrotising periodontal diseases. Med Oral Patol Oral Cir Bucal. 2004;9 Suppl:114-9; 08-14. [ Links ]
39. Heitz-Mayfield LJ. Systemic antibiotics in periodontal therapy. Aust Dent J. 2009;54 Suppl 1:S96-101. [ Links ]
40. Perusquia-Ortiz AM, Vazquez-Gonzalez D, Bonifaz A. Opportunistic filamentous mycoses: aspergillosis, mucormycosis, phaeohyphomycosis and hyalohyphomycosis. J Dtsch Dermatol Ges. 2012;10(9):611-21; quiz 21-2. [ Links ]
41. Deepa A, Nair BJ, Sivakumar T, Joseph AP. Uncommon opportunistic fungal infections of oral cavity: A review. J Oral Maxillofac Pathol. 2014;18(2):235-43. [ Links ]
42. Correa ME, Soares AB, de Souza CA, Cintra ML, Jorge J, Almeida OP, et al. Primary aspergillosis affecting the tongue of a leukemic patient. Oral Dis. 2003;9(1):49-53. [ Links ]
43. Fuqua TH, Jr., Sittitavornwong S, Knoll M, Said-Al-Naief N. Primary invasive oral aspergillosis: an updated literature review. J Oral Maxillofac Surg. 2010;68(10):2557-63. [ Links ]
44. Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58(2):281-97. [ Links ]
45. Scully C. Clinical practice. Aphthous ulceration. N Engl J Med. 2006;355(2):165-72. [ Links ]
46. Slebioda Z, Szponar E, Kowalska A. Recurrent aphthous stomatitis: genetic aspects of etiology. Postepy Dermatol Alergol. 2013;30(2):96-102. [ Links ]
47. Shiboski CH, Patton LL, Webster-Cyriaque JY, Greenspan D, Traboulsi RS, Ghannoum M, et al. The Oral HIV/AIDS Research Alliance: updated case definitions of oral disease endpoints. J Oral Pathol Med. 2009;38(6):481-8. [ Links ]
48. Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc. 2003;134(2):200-7. [ Links ]
49. Firth FA, Friedlander LT, Parachuru VP, Kardos TB, Seymour GJ, Rich AM. Regulation of immune cells in oral lichen planus. Arch Dermatol Res. 2015;307(4):333-9. [ Links ]
50. Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Scientific World Journal. 2014;2014:742-826. [ Links ]
51. Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145(1):45-56. [ Links ]
52. van der Waal I. Oral potentially malignant disorders: is malignant transformation predictable and preventable? Med Oral Patol Oral Cir Bucal. 2014;19(4):e386-90. [ Links ]
53. Eisen D, Carrozzo M, Bagan Sebastian JV, Thongprasom K. Number V Oral lichen planus: clinical features and management. Oral Dis. 2005;11(6):338-49. [ Links ]
54. Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 Suppl:S25 e1-12. [ Links ]
55. Jainkittivong A, Kuvatanasuchati J, Pipattanagovit P, Sinheng W. Candida in oral lichen planus patients undergoing topical steroid therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(1):61-6. [ Links ]
56. Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15(2):127-32. [ Links ]
57. Venables ZC, Narayana K, Johnston GA. Two unusual cases of allergic contact stomatitis caused by methacrylates. Contact Dermatitis. 2016;74(2):126-7. [ Links ]
58. Tremblay S, Avon SL. Contact allergy to cinnamon: case report. J Can Dent Assoc. 2008;74(5):445-61. [ Links ]
59. McCartan BE, McCreary CE. Oral lichenoid drug eruptions. Oral Dis. 1997;3(2):58-63. [ Links ]
60. Thornhill MH, Pemberton MN, Simmons RK, Theaker ED. Amalgam-contact hypersensitivity lesions and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):291-9. [ Links ]
61. Andrade P, Brinca A, Goncalo M. Patch testing in fixed drug eruptions--a 20-year review. Contact Dermatitis. 2011;65(4):195-201. [ Links ]
62. Gupta S, Gupta S, Mittal A, David S. Oral fixed drug eruption caused by gabapentin. J Eur Acad Dermatol Venereol. 2009;23(10):1207-8. [ Links ]
63. Benedix F, Schilling M, Schaller M, Rocken M, Biedermann T. A young woman with recurrent vesicles on the lower lip: fixed drug eruption mimicking herpes simplex. Acta Derm Venereol. 2008;88(5):491-4. [ Links ]
64. Savin JA. Current causes of fixed drug eruption in the UK. Br J Dermatol. 2001;145(4):667-8. [ Links ]
65. Batchelor JM, Todd PM. Allergic contact stomatitis caused by a polyether dental impression material. Contact Dermatitis. 2010;63(5):296-7. [ Links ]
66. Akhavan A, Alghaithi K, Rabach M, Mirchandani N, Cohen SR. Allergic contact stomatitis. Dermatitis. 2006;17(2):88-90. [ Links ]
67. Kind F, Scherer K, Bircher AJ. Allergic contact stomatitis to cinnamon in chewing gum mistaken as facial angioedema. Allergy. 2010;65(2):276-7. [ Links ]
68. Calapai G, Miroddi M, Mannucci C, Minciullo P, Gangemi S. Oral adverse reactions due to cinnamon-flavoured chewing gums consumption. Oral Dis. 2014;20(7):637-43. [ Links ]
69. Khatibi M, Shakoorpour AH, Jahromi ZM, Ahmadzadeh A. The prevalence of oral mucosal lesions and related factors in 188 patients with systemic lupus erythematosus. Lupus. 2012;21(12):1312-5. [ Links ]
70. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365-81. [ Links ]
71. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575-80. [ Links ]
72. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110-21. [ Links ]
73. Bafna Y, Khandelwal V, Bafna M, Nayak PA. Management of sublingual ulceration in a 12-month-old child. BMJ Case Rep. 2013;2013. [ Links ]
74. Baldiwala M, Nayak R. Conservative management of Riga-Fede disease. J Dent Child (Chic). 2014;81(2):103-6. [ Links ]
75. Scipio JE, Murti PR, Al-Bayaty HF, Matthews R, Scully C. Metastasis of breast carcinoma to mandibular gingiva. Oral Oncol. 2001;37(4):393-6. [ Links ]
76. Sonis ST. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009;45(12):1015-20. [ Links ]
77. Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46(6):452-6. [ Links ]
78. Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009;19(1):29-34. [ Links ]
 Correspondence:
Correspondence:
J Fourie
Department of Periodontics and Oral Medicine
Sefako Makgatho Health Sciences University
Po Box 230, Irene 0062.
Cell: 082 460 8368.
E-mail: drfouriej@gmail.com













