Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.10 Johannesburg Nov. 2016
RESEARCH
Effect of denture cleansers on flexural strength of heat-polymerized and auto-polymerized acrylic resins
RPN MorwengI; ARM EssopII; D MotlobaIII
IBDS. Department of Prosthodontics, School of Oral Health Sciences, Sefako Makgatho Hospital
IIAhmed RM Essop: BSc BDS MDent. Department of Prosthodontics, School of Oral Health Sciences, Sefako Makgatho Hospital
IIIBDS, MPH (Epid), MDent (Comm.Dent), MBL. Head, Department of Community Dentistry. School of Oral Health Sciences, Sefako Makgatho Health Sciences University
ABSTRACT
AIM: To compare the flexural strengths of two types of acrylic resins after immersion in three different denture cleansers, for two different time periods.
MATERIALS AND METHODS: 380 rectangular acrylic resin specimens (60mm x 20mm x 2mm) were fabricated and divided into three groups. Group 1 - baseline, ten of each type of acrylic resin; Group 2 - heat-polymerizing, 180 specimens, and Group 3 - auto-polymerizing, 180 specimens. Random samples of 30 specimens from Groups 2 and 3 were severally immersed in three different liquids: two denture cleansers i.e. alkaline peroxide- based (Corega) and sodium hypochloride- based (Jik), and tap water. Immersion time of six hours was taken to represent one day, hence, three and six months of continuous immersion represent one year and two years realtime, respectively. Flexural strengths were determined before, then after the three and six month periods. A two-way analysis of variance (SPSS version 23.0© (IBM USA) determined any statistical differences between the recorded flexural strengths.
RESULTS: Sodium hypochloride decreased flexural strength for both polymethylmethacrylate resins. Water reduced flexural strength of the heat-polymerizing resin. The alkaline peroxide cleaner had no impact on flexural strength.
CONCLUSION: Flexural strength of heat-polymerizing acrylic resin can be significantly reduced by exposure to denture cleansers.
Keywords: flexural strength, heat-polymerizing acrylic resin, self- polymerizing acrylic resin
INTRODUCTION
Acrylic resins are used in the fabrication of different types of dental prostheses. These resins are composed of polymethylmethacrylate (PMMA) or polyethylmethacrylate (PEMA) powder particles, a peroxide initiator, and pigments which are mixed with methacrylate monomers.1 The material most commonly used is PMMA,2 which does have limitations in terms of flexural strength, a measure of stiffness and resistance to fracture.3
PMMA resins may be divided into three types, based on the procedures to be used during processing i.e. heat- polymerization; dough auto-polymerization and pour-type auto-polymerization.3,4 All of these resins have low strength, are brittle on impact but, are fairly resistant to fatigue failure and are moderately flexible.5 The properties of the polymer network may be altered by absorption of water and/or chemical solutions. These alterations include changes in physical properties such as plasticization and softening as well as changes in chemical properties such as oxidation and hydrolysis.3 As reviewed by Ferracane,6 the extent of the effect on the polymer network is dependent upon the nature of the aqueous environment as well as the chemistry and structure of the resin.
During delivery of a dental prosthesis, patients are advised and given instructions on denture care. Apart from directions on regular brushing of the dentures, it is generally recommended that patients immerse the prosthesis in denture cleansers for variable periods of time.7 These instructions are intended to prolong the longevity of the prosthesis as well as to ensure the maintenance of a healthy state of the oral mucosa.8,9
Sodium hypochlorides (NaOCl) and the alkaline peroxides are the active ingredients in the two main classes of denture cleansers.9-11 Ideally, cleansers have antimicrobial properties, remove organic and inorganic biofilm, while having minimal effect on oral tissues.10 They should not alter the physical and chemical properties of the acrylic resin.12,13 Paranhos14 found that the chemical composition of cleansers and the immersion time, the contact period between the denture and the cleanser, play significant roles in changes in the acrylic resin over time. This study was undertaken to investigate the effects of denture cleansers and immersion periods on the flexural strengths of two different types of acrylic resins.
MATERIALS AND METHODS
(i )Preparation of specimens
Baseplate wax rectangular patterns of dimensions 60mm x 20mm x 2mm were prepared and invested in type II dental stone (Dentstone KD®) in metal flasks. After setting, the flask halves were separated, and the moulds rinsed under boiling water to remove the wax pattern. The heat-polymerized (Meliodent; Hanau, Germany) and auto-polymerized (Meliodent Rapid Repair; Hanau, Germany), specimens were fabricated separately, 190 of each acrylic type. The acrylic resin was mixed according to the manufacturer's instructions, was packed into the stone mould and flasked. After flasking the heat polymerizing specimens were subjected to in a thermostatically controlled water bath. The auto-polymerizing specimens were subjected to the process of polymerization in accord with the manufacturer's instructions.
(ii) Allocation of specimens
From each acrylic brand, 10 specimens were used to determine baseline values of flexural strength before immersion. A calibrated Instron material testing machine (model 3366), with a 1 KN load cell at a crosshead speed of 0.5mm/min was used for this determination, based on a three point bending test (Figure 1). The remaining 180 specimens were allocated in batches of thirty (30) to three different cleansers (Corega, NaOCl/Jig and water). Using an accelerated time protocol of six hours of immersion to represent one day, samples of each specimen type remained immersed in each cleanser for a period of either three months or six months, an equivalent of one and two years respectively. The flexural strengths of specimens (N/mm2) were measured after the three and six month periods.
(iii) Immersion of specimen in cleansers
Samples of 30 specimens each were immersed separately in one or other of the three different cleansers viz. the alkaline peroxide brand (Corega tablets, GlaxoSmithKline), the sodium hypochloride brand (household bleaching agent, Jik, Unilever), and tap water. The concentrations and amount of cleansers used were according to manufacturer's instructions. The tests were performed at room temperature, and cleansers were changed periodically to simulate daily immersion by the patient. Half the specimens remained immersed in their respective cleansers for three months, the remainder for six months, hence the procedure involved twelve Groups (two types of acrylic, three solutions, two time periods). At the end of the three month and the six month immersion periods, the Instron material testing machine was used to measure the flexural strength of each specimen (Figure 1). A single operator conducted the tests in order to minimise systematic error.
(iv) Data analysis
Data were collected and prepared in an electronic database for statistical analysis using SPSS version 23.0© (IBM USA). The effect of the independent variables, the period of immersion and the type of denture cleansers, on the flexural strength were determined by a two-way analysis of variance (ANOVA). Having completed the ANOVA, post hoc testing was enabled, and in the case of significant differences, multiple comparisons were undertaken using Tukey's HSD test. Hypothesis testing was set at α=0.05.
RESULTS
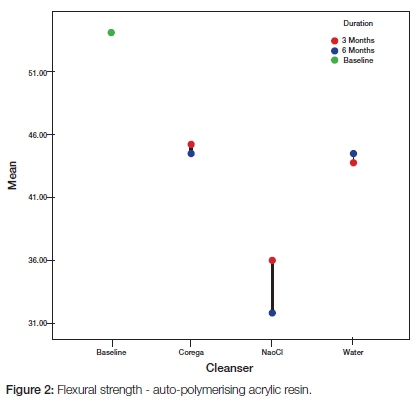
All groups demonstrated a decrease in flexural strength for all cleansers and for both immersion periods. Compared with the baseline values, the effects of Corega and of water on the flexural strengths of auto-polymerizing acrylic were almost equal whilst the hypochloride solution exerted a more dramatic effect resulting in a greater loss of flexural strength (Figure 2). Further, the difference in flexural strengths from the three month period to the six month period showed little change for the Corega and water samples, but there was a marked continued loss over that period in the flexural strengths of the sample which had been immersed in the hypochloride solution (Figure 2).
The heat-polymerizing acrylic samples showed a similar tendency with the hypochloride solution being seen to have resulted in much greater loss of structural strength than was the effect seen with the Corega based sample (Figure 3). Immersion in water, however, produced a loss which at the three month period was even greater than that experienced by the acrylic soaked in hypochloride (Figure 3).
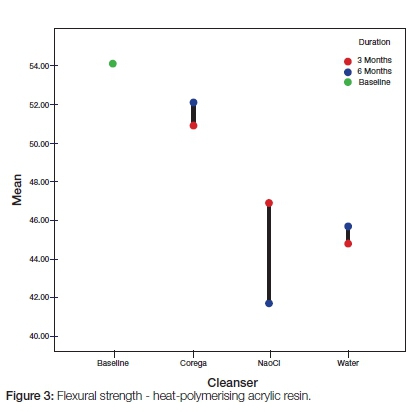
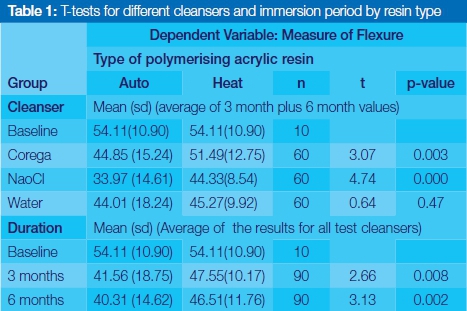
Immersion in the chemical cleansers caused a significantly greater reduction of flexural strength for auto-polymerizing acrylic resin compared with the effect on heat-polymerizing acrylic resin (Corega: p=0.003 and NaoCL: p=0.000) (Students t test, significance set at p=0.05, Table 1). All three test solutions were found to have deleteriously affected flexural strengths the longer the immersion time, so that measurements of the strengths at the six month period were lower than at the three month stage (Figures 2 and 3).3 Statistical t test comparison of data related to immersion periods showed significant differences between the weakening of auto- and heat-polymerizing acrylics between three and six months when compared with the baseline data (p=0.008 and 0.002), with the auto-polymerizing sample showing greater loss (Table 1).
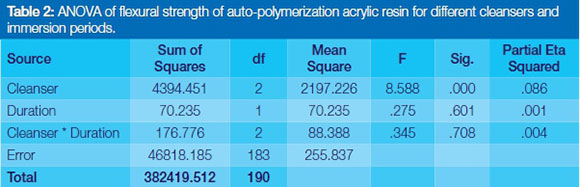
A two-way analysis of variance (ANOVA) between the data from the Groups was conducted to explore the impact of the cleansers and the duration of immersion (the independent variables) on the flexural strengths (the outcome variable). For both auto-polymerizing and heat-polymerizing acrylic resins, there was a statistically significant main effect only for cleansers [F (2,183) =8.588, p=0.000; F (2,183) =8.185, p=0.000].(Tables 2 and 3). However it was found following the Tukey HSD posthoc tests that the effect sizes were small (Partial Eta squared =0.086 and 0.082, Tables 2 and 3) respectively. (Effect size [measured as Partial Eta squared] is the size of the difference between groups).

DISCUSSION
Acrylic resins have different physical and mechanical properties, hence the marked differences in how they react when exposed to diverse environments such as water, disinfectants and denture cleansers.2,10,12 Repetitive masticatory forces on dentures cause flexural fatigue of the acrylic denture base. Hence a prosthesis should be fabricated with denture base material of high flexural strength in order to withstand these loads. The flexural strength is indicative of the compressive, tensile and shear strengths, which translates as stiffness and resistance of a material to fracture.10 Therefore flexural strength determines the longevity and success of a denture.15 To minimise fractures, PMMA resins are reinforced with fibres, glass and aramid.16-18 Similarly, several processing techniques have been introduced to increase fatigue strength.10 The consensus is that heat-polymerising acrylic resins have superior flexural strength compared with auto-polymerizing resins.19,20 The latter are susceptible to porosities and deform relatively easily under load, leading to high rates of fracture.20
Denture cleansers remove debris on denture surfaces, and for individuals with impaired dexterity the use of these chemicals is highly recommended.21,22 Inappropriate choice and use of chemical cleansers can, however, cause damage to dentures.23,24 Guidance on the selection of cleanser is therefore of high importance.
The study concluded that both acrylic resins demonstrated a decline in flexural strength when immersed in denture cleansers. The findings concur with results from Peracini20 and Pisani13 who observed that heat-polymerising resins are more prone to fracture after having been immersed in cleansers. The results of the current study also show that auto-polymerising acrylic resins are more adversely affected by chemical cleaners than heat-polymerising resin. It is hypothesised that the very high levels of porosity observed in auto-polymerising resin reduce their flexural strength and weaken the resin. Other effects of porosity include compromised aesthetic properties and increased propensity to harbour and promote growth of Candida in these dentures. Ultimately inflammation and soreness of the soft tissues occur underneath the denture.
The study showed that hypochloride cleansers have greater detrimental effect on the flexural strength of both types of acrylic resins than either Corega or water, a finding supported in the literature.13,14,24 IT has also been shown, however, that at 0.5% concentration, sodium hypochloride cleansers cause no structural changes to the dentures, but do provide clinically effective antibacterial and antifungal properties.10,25 The increase in concentration above 0.5% results in discolouration, denture roughness and structural weakness.21,25 Extended use of low concentration sodium hypochloride cleansers could possibly produce similar outcomes as high dosage cleansers. Corega is effective in the removal of biofilm, but has no significant effect on the flexural strength of the resins, irrespective of duration of immersion. Water does not provide chemical therapeutic advantage,8,26 but impacts negatively on the flexural strength of heat-polymerizing acrylic resin.
CONCLUSION
Given the limitations of this study, it is concluded that denture cleansers have an effect on the flexural strength of polymethylmethacrylate resins. Specifically, sodium hypochloride cleansers will result in significant reductions in the flexural strength of acrylic resins after prolonged exposure. Similarly, this study showed that immersion of heat-polymerizing acrylic resin in water for six months weakens the acrylic. Corega remains the most effective cleanser to use for denture care.
IMPLICATIONS FOR CLINICIANS
Generally patients who receive dentures are advised to keep the dentures in water for a period of at least six hours or overnight. However this study has shown that immersion in water overnight for long durations reduces the flexural strength of the acrylic resins. Therefore it is important that patients are advised to refrain from this practice. It is also important, however, to maintain a moist environment for the dentures when they are not in use, hence the suggestion that the dentures must be wrapped in a wet paper towel and not immersed completely in water when not being worn.
The use of denture cleansers to maintain dentures free of pathogens may be necessary only after meals and not require immersion overnight or for long durations. Where patients elect to immerse dentures in a cleanser, it preferable to use Corega; immersion in water should be done for shorter periods. Sodium hypochloride cleansers can be used for cleaning the dentures without extended periods of immersion in the cleanser. Patients should also be reminded to use a toothbrush to clean the dentures manually.
ACRONYMS
PEMA: polyethylmethacrylate
PMMA: polymethylmethacrylate
References
1. Bettencourt AF, Neves CB, de Almeida MS, et al. Biodegradation of acrylic based resins: A review. Dental Materials. 2010;26(5):e171-e180. [ Links ]
2. Bertassoni LE, Marshall GW, de Souza EM, Rached RN. Effect of pre-and postpolymerization on flexural strength and elastic modulus of impregnated, fiber-reinforced denture base acrylic resins. The Journal of Prosthetic Dentistry. 2008;100(6):449-57. [ Links ]
3. Ruyter IE, Svendsen SA. Flexural properties of denture base polymers. The Journal of Prosthetic Dentistry. 1980;43(1):95-104. [ Links ]
4. Moosa R, Ghani F. Effect of curing methods and temperature on porosity in acrylic resin bases.. J Pak DentAssoc. 2012;21(3):127-35. [ Links ]
5. Palitsch A, Hannig M, Ferger P, Balkenhol M. Bonding of acrylic denture teeth to MMA/PMMA and light-curing denture base materials: the role of conditioning liquids. Journal of Dentistry. 2012;40(3):210-21. [ Links ]
6. Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22(3):211-22. [ Links ]
7. Gajwani-Jain S, Magdum D, Karagir A, Pharane P. Denture cleansers: A review. IOSR Journal of Dental and Medical Sciences. 2015;1(14):94-6. [ Links ]
8. Cruz PC, Andrade IMd, Peracini A, et al. The effectiveness of chemical denture cleansers and ultrasonic device in biofilm removal from complete dentures. Journal of Applied Oral Science. 2011;19(6):668-73. [ Links ]
9. Salles MM, Badaro MM, ARRUDA CNFd. Antimicrobial activity of complete denture cleanser solutions based on sodium hypochlorite and Ricinus communis-a randomized clinical study. Journal of Applied Oral Science. 2015;23(6):637-42. [ Links ]
10. Pahuja RK, Garg S, Bansal S, Dang RH. Effect of denture cleansers on surface hardness of resilient denture liners at various time intervals-an in vitro study. TheJournal of Advanced Prosthodontics. 2013;5(3):270-7. [ Links ]
11. Vojdani M, Kohanteb J, Negabat N. Comparision of the effect of three denture cleansers on prosthetic Microorganisms. Journal of Dentistry, Shiraz University of Medical Sciences. 2002;3(3, 4):61-9. [ Links ]
12. Felipucci DNB, Davi LR, Paranhos HFO, Bezzon OL, Silva RF, Pagnano VO. Effect of different cleansers on the surface of removable partial denture. Brazilian Dental Journal. 2011;22(5):392-7. [ Links ]
13. Pisani MX, Macedo AP, Paranhos HdFO, Silva CHLd. Effect of experimental Ricinus communis solution for denture cleaning on the properties of acrylic resin teeth. Brazilian Dental Journal. 2012;23(1):15-21. [ Links ]
14. Paranhos HdFO, Peracini A, Pisani MX, Oliveira VdC, Souza RFd, Silva-Lovato CH. Colour stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Brazilian Dental Journal. 2013;24(2):152-6. [ Links ]
15. Diaz-Arnold AM, Vargas MA, Shaull KL, Laffoon JE, Qian F. Flexural and fatigue strengths of denture base resin. The Journal of Prosthetic Dentistry. 2008;100(1):47-51. [ Links ]
16. Ajaj-ALKordy NM, Alsaadi MH. Elastic modulus and flexural strength comparisons of high-impact and traditional denture base acrylic resins. The Saudi Dental Journal. 2014;26(1):15-8. [ Links ]
17. John J, Gangadhar SA, Shah I. Flexural strength of heat-polymerized polymethyl methacrylate denture resin reinforced with glass, aramid, or nylon fibers. The Journal of Prosthetic Dentistry. 2001;86(4):424-7. [ Links ]
18. Ayaz EA, Durkan R. Influence of acrylamide monomer addition to the acrylic denture-base resins on mechanical and physical properties. International Journal of Oral Science. 2013;5(4):229-35. [ Links ]
19. Machado C, Sanchez E, Azer SS, Uribe JM. Comparative study of the transverse strength of three denture base materials. Journal of Dentistry. 2007;35(12):930-3. [ Links ]
20. Peracini A, Davi LR, de Queiroz Ribeiro N, de Souza RF, da Silva CHL, Paranhos HdFO. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. Journal of Prosthodontic Research. 2010;54(2):78-83. [ Links ]
21. Geramipanah F, Zeighami S. Effect of denture cleansers on tensile bond strengths of soft liners to denture base resin. Journal of Islamic Dental Association of Iran. 2013;25(2):172-9. [ Links ]
22. Sato S, Cavalcante MRS, Orsi IA, Paranhos HdFO, Zaniquelli O. Assessment of flexural strength and colour alteration of heat-polymerized acrylic resins after simulated use of denture cleansers. Brazilian Dental Journal. 2005;16(2):124-8. [ Links ]
23. Nikawa H, Hamada T, Yamashiro H, Kumagai H. A review of in vitro and in vivo methods to evaluate the efficacy of denture cleansers. International Journal of Prosthodontics. 1999;12(2):153-9. [ Links ]
24. Orsi IA, Andrade VG. Effect of chemical disinfectants on the transverse strength of heat-polymerized acrylic resins submitted to mechanical and chemical polishing. The Journal of Prosthetic Dentistry. 2004;92(4):382-8. [ Links ]
25. Subrata G. Antifungal properties of sodium peroxide and sodium hypochlorite as a denture cleanser for full acrylic denture in vitro. Padjadjaran J Dent. 2008;20(1):1-10. [ Links ]
26. Nikawa H, Iwanaga H, Hamada T, Yuhta S. Effects of denture cleansers on direct soft denture lining materials. The Journal of Prosthetic Dentistry. 1994;72(6):657-62. [ Links ]
 Correspondence:
Correspondence:
Refilwe PN Morweng
E-mail: Refilwe.Morweng-Mokgatlha@smu.ac.za













