Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.9 Johannesburg Oct. 2016
RESEARCH
Smear layer removal ability and antibacterial activity of endodontic irrigants
KR BennieI; CP OwenII; FS BothaIII
IDepartment of Oral Rehabilitation, School of Oral Health Science, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Oral Rehabilitation, School of Oral Health Science, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIDepartment of Paraclinical Sciences, Phytomedicine Programme, Faculty of Veterinary Science, University of Pretoria, Onderstepoort Campus, Pretoria, South Africa
ABSTRACT
INTRODUCTION: a variety of endodontic irrigants are available for endodontic irrigation. Irrigants must be effective in removing the smear layer created during endodontic therapy as well as eliminating bacteria.
AIM: This in vitro study tested various alternating sequences of sodium hypochlorite, anolyte solution (electrochemically activated water), and EDTA for their ability to do this.
METHOD: Forty-eight single canal teeth were randomly divided into six groups, prepared to working length, sterilised and inoculated with Enterococcus faecalis. Each group was assigned a different sequence of irrigants. Standard cultivation techniques were used to count the colony-forming units at each phase. Two SEM photomicrographs of each root's coronal, middle and apical thirds were taken randomly and the number of patent dentinal tubules counted. Statistical analysis was completed using One-way-ANOVA and multiple comparisons.
RESULTS: Group 6 (n=10) protocol of 5ml anolyte followed by 3ml 18% EDTA showed the best smear layer removal results for all thirds of the canal. Chemical irrigation significantly decreased the intracanal E. faecalis CFUs.
CONCLUSION: Within the limitations of the study anolyte solution followed by EDTA showed the best smear layer removal. The various sequences of NaOCl, anolyte solution, and EDTA all had similar antibacterial results.
Keywords: Antibacterial activity; EDTA; Electrochemically activated water; Irrigants; Smear Layer; Sodium hypochlorite.
Smear layer removal ability and antibacterial activity of endodontic irrigants.
INTRODUCTION
Endodontic treatment aims at eliminating microorganisms from the infected root canal system by mechanical and chemical methods.1 Mechanical preparation of the canals leads to the formation of a smear layer. This is an amorphous layer of unpredictable volume, comprising remnants of pulpal tissues, micro-organisms and debris from canal preparation.2,3 The smear layer should be removed as it may act as a substrate for remaining bacteria. Removal improves the seal of the root canal filling materials, reduces microleakage and improves the mechanical retention of the filling material to dentine.4-8
Bacteria remaining in the canals may penetrate the dentinal tubules as deep as 150μm in the apical two thirds of the canal and up to 400μm in the rest of the canal.9,10Enterococcus faecalis is a bacterium commonly associated with persisting endodontic disease and in secondary infections.11E. faecalis is able to survive harsh environments due to its high virulence. Within the root canal system it can bind to dentine as it possesses serine protease, gelatinases and collagen binding protein.12
Sodium hypochlorite is a well-established endodontic irrigant with the ability to dissolve tissues and exert an antibacterial effect.13,14 There is some controversy over the concentration to be used in endodontic irrigation, but Clegg et al. (2006)15 found that 6% sodium hypochlorite was the only concentration able to remove the biofilm and render the bacteria nonviable. Sodium hypochlorite is toxic to tissues and has been shown to reduce polymerisation of resin sealers such as Epiphany (Sybron Endo, Orange, CA, USA).16 Furthermore, it is unable to remove the smear layer and is corrosive to endodontic instruments.2,17-19
These negative characteristics of sodium hypochlorite warrant the search for a replacement.2,13
Although EDTA has a chelating action that assists in creating smear free dentine by dissolving mineralised tissues,20 sodium hypochlorite followed by EDTA did not produce complete smear layer removal.21 Anolyte solution has been suggested as a replacement irrigant.22 It does not produce a smear layer and in fact has been shown to remove any existing smear layer and exposed collagen fibrils.23 Anolyte solution, however, is not as antibacterial as sodium hypochlorite.18
It would thus seem reasonable to test further combinations of these irrigants for their effectiveness in removing the smear layer. The aim of this study was, therefore, to test various alternating sequences of sodium hypochlorite, anolyte solution, and EDTA for their ability to remove the mineralised portion of the smear layer, and to eliminate bacteria.
METHODS
Ethical clearance was obtained for the use of extracted teeth as well as for the use of E. faecalis (clearance number M050760). Pre-operative radiographs were taken of each tooth specimen. Teeth that had root fractures, multiple canals, complicated canal forms and/or pulp stones or calcifications were excluded from the study. Forty-eight single-canal teeth were selected. They were decoronated at the level of the cemento-enamel junction and the roots cleaned of any deposits using curettes. The canals of each root were explored using a 10 K hand file (Mani, Inc., Utsunomi Ya, Tochigi, Japan). The working length was established by piercing the apex of the canal until the file was just visible at the canal apex, and 0.5 mm was subtracted from this length. A glide file path was prepared using 10 K and 15 K hand files. Thereafter the roots were prepared using ProTaper nickel titanium rotary files (Dentsply, Maillefer, Baillaigues, Switzerland) in an endodontic handpiece according to the manufacturer's instructions. The canals were prepared using S1 and S2 files, followed by a 20 K hand file, F1 rotary file, 25 K hand file and finally the F2 rotary file. Between each file, and as often as additionally necessary, the canals were rinsed with sterile distilled water. The apices of the roots were sealed with GC Fuji I (GC Corporation, Tokyo, Japan) and the orifices with EcoTemp (Ivoclar Vivadent, New York, USA) (Lot J15944) to isolate the internal environment.
The teeth were randomly divided into six groups and placed in sterile Ringer's solution for 72 hours. Four groups contained 10 roots each (test) and two groups (controls) contained four roots each. The Ringer's solutions were replaced at 24 hour intervals. The roots were sonified three times and sterilized at 121°C for 15 minutes in an autoclave. In order to maintain sterile conditions the study was conducted in a positive sterile airflow laboratory, working in a laminar flow cabinet, using sterile gloves, masks and instruments. The 48 roots were placed in sterile bottles containing Casein-peptone Soymeal-peptone Broth (CASO Broth, Merck SA (Pty) Ltd., Halfway House, South Africa) and anaerobically incubated using Anaerocult A® (Merck SA (Pty) Ltd., Halfway House, South Africa) at 37°C for three days. Sterile paper points were inserted into the canals, then placed onto CASO Agar plates and incubated anaerobically using Anaerocult A® at 37°C for 72 hours. Negative cultures confirmed that the roots were sterile and did not contain any anaerobic bacteria before the inoculation procedure.
A MacFarland Standard-I suspension (8 x 108 colony-forming units [CFU]) of E. faecalis (ATCC49474) was prepared. A 1% suspension was added to the Broth and incubated anaerobically using Anaerocult A® at 37˚C for three days. The roots were sampled by inserting sterile paper points in the canals to soak up the inoculated broth from the canals. Each paper point was placed into a vial containing 0.9ml sterile Ringers solution. This served as a 1:10 dilution from which serial dilutions were made.
One hundred micro litres of each suspension was spread onto CASO-Agar plates in triplicate by means of the standardised glass spreading technique to quantify CFUs.19 The roots were then irrigated for one minute for each irrigant according to the following protocols:
Group 1: (n=4) 3ml sterile distilled water; Group 2: (n=4) 3ml 6% sodium hypochlorite; Group 3: (n=10) 3ml 6% sodium hypochlorite followed by 3ml 18% EDTA; Group 4: (n=10) 3ml 6% sodium hypochlorite followed by 5ml anolyte solution; Group 5: (n=10) 0.5ml 6% sodium hypochlorite followed by 5ml anolyte solution followed by 3ml 18% EDTA; Group 6: (n=10) 5ml anolyte solution followed by 3ml 18% EDTA.
Thereafter the irrigants were rinsed out of the canals with 10 ml of sterile distilled water. The irrigants were all delivered using a syringe (Ultradent Inc., South Jordan, USA) and 27 gauge Endo-EZE 1" Irrigator Tip (Ultradent, Inc., USA) to within 2 mm of the canal apex. The same cultivation technique described above was used after irrigation and the CFUs of bacteria that survived the irrigation process were quantified. The percentage difference between the CFUs before and after irrigation was calculated for each group and compared using the t-test (Statistical Package and Service Solutions (SPSS) Inc, Chicago, USA). A p-value ≤ 0.05 indicated a significant statistical difference at a 95% confidence interval. The inter-group percentage differences were compared using One-way ANOVA. Multiple comparisons were made using Tukey HSD or the Tamhane test depending on the normality of the data.
The roots were prepared for Scanning Electron Microscopy according to standard methods.24,25 Two photomicrographs were taken per third per tooth. This was done by superimposing a numbered grid over the relevant third and selecting random numbers from a statistical random number table. The selected block was magnified to 2500x. Using Image J software (U.S. National Institutes of Health, Bethesda, Maryland, USA) the open tubules in each photomicrograph were counted independently by two calibrated expert examiners. Partially open and closed tubules were not counted. Open tubules were defined as round, with no smear layer or matter overlying the tubule opening. Bacteria may be present inside the tubule such that a sealer will entomb it when penetrating the canal but may not be covering the opening. Inter-rater reliability was determined using the kappa-test. Where the examiners differed, consensus was reached after discussion. The One-way ANOVA test was used to establish intra-group and inter-group differences. A p-value ≤ 0.05 indicated a significant statistical difference at a 95% confidence interval. The Tukey HSD or Tamhane test was used for multiple comparisons.
RESULTS
Multiple comparisons showed statistically significant differences (p<0.05) between Group 6 and all the other groups, and between Group 5 and Group 1 for the coronal third. There were statistically significant differences (p<0.05) between Group 6 and Groups 1-4 for the middle third (Figure 1).
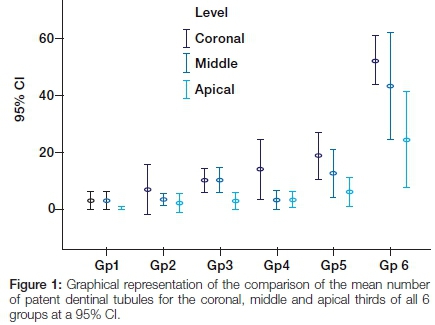
In the comparison of the apical thirds a statistical anomaly occurred. The one-way ANOVA showed a statistical difference but multiple comparisons failed to show where the differences were. The examiners pointed out that there was a marked visual difference observed between Group 6 and all other groups. Group 6 presented with a thinner smear layer and a larger number of patent dentinal tubules compared with other groups. The latter demonstrated thick smear layers that completely or partially covered the dentinal tubules and inter-tubular dentine.
Intra-group comparisons showed statistically significant differences in the CFUs before and after irrigation for all groups. Inter-group comparisons showed statistically significant results (p=0.000). Multiple comparisons revealed only a statistically significant difference (p<0.05) between Group 1 (sterile water) and all other groups.
Figures 2 to 4 show SEM photomicrographs (at 2500x) of the middle third of the canal. Figure 2 is of a Group 3 root after irrigation with 6% sodium hypochlorite followed by 18% EDTA. A regular distribution of open and partially open dentinal tubules can be seen (white arrow). Patches of flat smear layer are seen over a few tubules and on the intertubular dentine (black arrow).
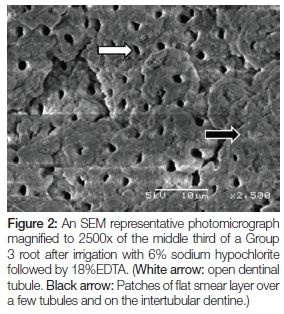
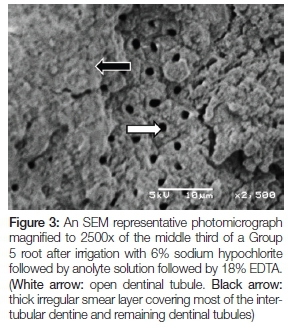
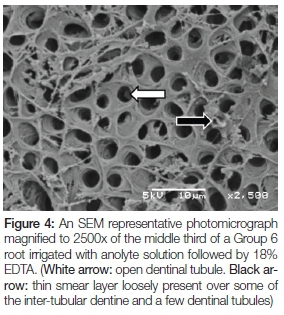
Figure 3 is of a Group 5 root after irrigation with 6% sodium hypochlorite followed by anolyte solution followed by 18% EDTA. A low to moderate number of dentinal tubules are open (white arrow). A thick irregular smear layer can be seen covering most of the intertubular dentine and remaining dentinal tubules (black arrow).
Figure 4 is of a Group 6 root irrigated with anolyte solution followed by 18% EDTA. Regularly distributed open dentinal tubules can be seen (white arrow). A thin smear layer is loosely present over some of the inter-tubular dentine and a few dentinal tubules (black arrow).
DISCUSSION
Statistical Analysis
Previous studies have analysed the smear layer removal ability of irrigants by semi-quantitative methods. This included assessing each photomicrograph and scoring the smear layer removal on a scale.26 In this study the actual number of open dentinal tubules was counted in each photomicrograph in order to reduce the possibility of subjective analyses. Due to the variable nature of dentine this quantitative analysis may have allowed for a larger standard deviation than that observed with semi-quantitative analyses.
Smear layer removal
Where sodium hypochlorite was the sole irrigant there was a thick irregular smear layer that was structurally different to that observed in the roots irrigated with sterile distilled water. This is in agreement with other research that has shown sodium hypochlorite cannot remove the inorganic portion of the smear layer.2,18
For all other irrigant sequences there was better smear layer removal in the coronal third. This may be because the irrigation solution did not reach the apical and possible middle third due to an operator error, or insufficient canal preparation. Histological differences in dentine may have also affected the smear layer removal in the apical thirds. Smear layer removal may have been improved with increased contact time, more frequent replacement or activation of the irrigants.27-31
Alternating the use of a tissue solvent (sodium hypochlorite) and a chelating agent (EDTA) improved smear layer removal. Alternating sodium hypochlorite with anolyte solution showed a visual trend toward improved smear layer removal compared with sodium hypochlorite alone. This was demonstrated by an increased number of patent dentinal tubules. Sodium hypochlorite produced a thick smear layer, which completely covered the dentinal tubules and inter-tubular dentine. This may indicate the role of the anolyte solution in smear layer removal.26-29
No one group was able to completely remove the smear layer in all thirds, but where anolyte solution was followed by 18% EDTA there was improved smear layer removal compared with other groups. Thus, anolyte solution followed by EDTA may be a promising irrigation protocol. Further research is required to establish the ideal volume, contact time and irrigation method.
Antibacterial activity
Where 3 ml of the sodium hypochlorite was used (Groups 2, 3 and 4), the CFU count after irrigation was always zero. Thus 3 ml of 6% sodium hypochlorite with surfactant molecules used for one minute was effective against E. faecalis under the conditions of this study.
In Group 5 (sodium hypochlorite followed by anolyte solution followed by EDTA) and Group 6 (anolyte solution followed by EDTA) the CFU count after irrigation was so close to zero that the percentage difference before and after irrigation was deemed statistically insignificant compared with the groups that had a zero CFU count (Groups 2, 3 and 4). Statistically the CFU after irrigation may be deemed insignificant but clinically the remaining microorganisms in Groups 5 and 6 cannot be discounted. Some authors have suggested that any remaining bacteria that are not entombed in the dentinal tubules during obturation may potentially multiply and migrate apically leading to failure of the endodontic treatment.32,33E.faecalis is particularly virulent and may survive for long periods with little or no substrate.34-36 Group 1 (sterile distilled water) had the highest CFUs after irrigation and the percentage difference was deemed statistically significant compared with other groups. This indicates that although chemical irrigation does significantly reduce the intracanal CFU count, an antibacterial irrigant is more effective.
The limitations of this in vitro study include a small sample size and the use of cultivation techniques which may not be as sensitive to other remaining microbial species.
CONCLUSIONS
Within the limitations of this study, the results indicate the following:
• For any irrigant group better smear layer removal was shown in the coronal third than in the apical third.
• 5ml anolyte solution followed by 3ml 18% EDTA for one minute showed the best smear layer removal results for all thirds.
• Chemical irrigation significantly decreases the intracanal E. faecalis CFUs.
• Sterile distilled water is not effective in decreasing the intracanal CFUs.
• All other irrigant protocols were equally antibacterial.
Acknowledgments
This study was partially funded with a grant from the Faculty Research Committee of the Faculty of Health Sciences, University of the Witwatersrand.
References
1. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89(4):321-8 [ Links ]
2. McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1(7):238-42 [ Links ]
3. Ballal NV, Kandian S, Mala K, Bhat KS, Acharya S. Comparison of the efficacy of maleic acid and ethylenediaminetetra-acetic acid in smear layer removal from instrumented human root canal: a scanning electron microscopic study. J Endod. 2009;35(11):1573-6 [ Links ]
4. White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J Endod. 1984;10(12):558-62 [ Links ]
5. Okşan T, Aktener BO, Sen BH, Tezel H. The penetration of root canal sealers into dentinal tubules. A scanning electron microscopic study. Int Endod J. 1993;26(5):301-5 [ Links ]
6. Behrend GD, Cutler CW, Gutmann JL. An in-vitro study of smear layer removal and microbial leakage along root-canal fillings. Int Endod J. 1996;29(2):99-107 [ Links ]
7. Vivacqua-Gomes N, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Influence of irrigants on the coronal microleakage of laterally condensed gutta-percha root fillings. Int Endod J. 2002;35(9):791-5 [ Links ]
8. Clark-Holke D, Drake D, Walton R, Rivera E, Guthmiller JM. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J Dent. 2003;31(4):275-81 [ Links ]
9. Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66(8):1375-9 [ Links ]
10. Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol . 1995;11(1):6-9 [ Links ]
11. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 1998;85(1):86-93 [ Links ]
12. Hubble TS, Hatton JF, Nallapareddy SR, Murray BE, Gillespie MJ. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol Immunol . 2003;18(2):121-6 [ Links ]
13. Shih M, Marshall FJ, Rosen S. The bactericidal efficiency of sodium hypochlorite as an endodontic irrigant. Oral Surg Oral Med Oral Pathol . 1970;29(4):613-9 [ Links ]
14. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15(4):187-96 [ Links ]
15. Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J Endod. 2006;32(5):434-7 [ Links ]
16. Nunes VH, Silva RG, Alfredo E, Sousa-Neto MD, Silva-Souso YT. Adhesion of Epiphany and AH Plus sealers to human root dentin treated with different solutions. Braz Dent J. 2008;19(1):46-50 [ Links ]
17. Neal RG, Craig RG, Powers JM. Effect of sterilization and irrigants on the cutting ability of stainless steel files. J Endod. 1983;9(3):93-6 [ Links ]
18. Marais JT, Williams WP. Antimicrobial effectiveness of electro-chemically activated water as an endodontic irrigation solution. Int Endod J. 2001;34(3):237-43 [ Links ]
19. Gernhardt CR, Eppendorf K, Kozlowski A, Brandt M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004;37(4):272-80 [ Links ]
20. Ciucchi B, Khettabi M, Holz J. The effectiveness of different endodontic irrigation procedures on the removal of the smear layer: a scanning electron microscopic study. Int Endod J. 1989;22(1):21-8 [ Links ]
21. Lim TS, Wee TY, Choi MY, Koh WC, Sae-Lim V. Light and scanning electron microscopic evaluation of Glyde File Prep in smear layer removal. Int Endod J. 2003;36(5):336-43 [ Links ]
22. Solovyeva AM, Dummer PM. Cleaning effectiveness of root canal irrigation with electrochemically activated anolyte and catholyte solutions: a pilot study. Int Endod J. 2000;33(6):494-504 [ Links ]
23. Marais JT. Cleaning efficacy of a new root canal irrigation solution: a preliminary evaluation. Int Endod J. 2000;33(4):320-5 [ Links ]
24. Glauert, A.M. 1975. Fixation, dehydration and embedding of biological specimens. In: Practical Methods in Electron Microscopy. North-Holland Publishing, Amsterdam [ Links ]
25. Hayat, M.A. 1981. Principles and Techniques of Electron Microscopy, Biological Applications. 1. University Park Press, Baltimore [ Links ]
26. Garcia F, Murray PE, Garcia-Godoy F, Namerow KN. Effect of aquatine endodontic cleanser on smear layer removal in the root canals of ex vivo human teeth. J Appl Oral Sci. 2010;18(4):403-8 [ Links ]
27. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg. 1983;55(3):307-12 [ Links ]
28. Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions :Part 3. J Endod. 1983;9(4):137-42. [ Links ]
29. Calt S, Serper A. Smear layer removal by EGTA. J Endod. 2000;26(8):459-61 [ Links ]
30. Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27(7):452-5 [ Links ]
31. Scelza MF, Pierro V, Scelza P, Pereira M. Effect of three different time periods of irrigation with EDTA-T, EDTA, and citric acid on smear layer removal. Oral Surg Oral Med Oral Pathol Oral Radiol . 2004;98(4):499-503 [ Links ]
32. Byström A, Happonen RP, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol. 1987;3(2):58-63 [ Links ]
33. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30(5):297-306 [ Links ]
34. Molander A, Reit C, Dahlén G. The antimicrobial effect of calcium hydroxide in root canals pretreated with 5% iodine potassium iodide. Endod Dent Traumatol . 1999;15(5):205-9 [ Links ]
35. Love RM. Enterococcus faecalis - a mechanism for its role in endodontic failure. Int Endod J. 2001;34(5):399-405 [ Links ]
36. Fidgor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol . 2003;18(4):234-9 [ Links ]
 Correspondence:
Correspondence:
C Peter Owen
Professor Emeritus
Department of Oral Rehabilitation
School of Oral Health Science
Faculty of Health Sciences
University of the Witwatersrand
7 York Road, Parktown 2193
Cell: +27 83 679 2205.
Fax: +27 86 553 4800.
Email: peter.owen@wits.ac.za













