Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.71 no.8 Johannesburg Set. 2016
RESEARCH
The use of textural analysis to test the hardness and penetrability of three types of gutta percha cones when exposed to two endodontic solvents
E PatelI; C P OwenII
IBDS, MScDent. Lecturer, Division of Operative Dentistry and Endodontics, Department of Oral Rehabilitation, School of Oral Health Science, Faculty of Health Sciences, University of the Witwatersrand
IIBChD, MScDent, MChD, FICD, FCD(SA). Professor Emeritus, Department of Oral Rehabilitation, School of Oral Health Science, Faculty of Health Sciences, University of the Witwatersrand
ABSTRACT
AIM: Gutta-percha (GP) is removed from root canals by mechanical instrumentation used in conjunction with solvents such as Xylene and Eucalyptus oil. This study used textural analysis to test changes in the penetrability and hardness of Conventional GP, Thermafil® and Guttacore™ when exposed to these solvents: rigidity was used for hardness and deformation energy and resilience for penetrability.
METHODS: GP cones (n=81) were tested prior to, and following, solvent exposure. For each outcome variable, results were tabulated by group. Between-group differences were assessed employing a General Linear Model, with the outcome as the dependent variable and the solvent, GP type and solvent-GP type interaction as the independent variables.
RESULTS: Significant differences in rigidity and deformation energy were observed. Resilience decreased in Thermafil and Guttacore, but increased in Conventional GP. A greater reduction in the hardness of Thermafil was observed with Eucalyptus oil. Conventional GP was susceptible to both solvents but penetrability decreased with Xylene. Guttacore was significantly altered by both solvents.
CONCLUSIONS: Considering the toxicity profile of Xylene, and the biocompatibility and antimicrobial effects of Eucalyptol, Eucalyptus oil is recommended for use during endodontic retreatment.
Keywords: Gutta percha; hardness; penetrability; solvents
INTRODUCTION
An important stage in endodontic therapy is the three-dimensional filling of the root canal system to provide as perfect a seal as possible to aid periapical repair.1,2 Since its introduction as a root filling material by Bowman in 1867, gutta-percha (GP) has remained the material of choice, and has thus been synonymous with endodontic obturation.3 Endodontic therapy is dependent on multiple factors, and, even when meticulously performed, can fail, resulting in the need for retreatment.4,5
An important objective of endodontic retreatment is the removal of the GP filling material from the canal(s) to regain access to the apical foramen or foramina.6 Whilst mechanical instrumentation serves as the primary method of GP removal, many studies have shown that this alone is insufficient, for it allows residual GP material to remain in the canal.7,8 Thus, chemical solvents were proposed to serve as adjuncts to mechanical removal.9 These solvents soften and partially dissolve GP, rendering it more amenable to removal with mechanical instruments, thereby decreasing the risk of perforation.10
Advances in endodontic retreatment have also lead to changes in the solvents used. Chloroform and Halothane were the solvents of choice for many years as they were the most effective in dissolving endodontic sealants.11,12 However, due to the related toxicity and carcinogenicity of these solvents, clinicians have sought suitable alternatives.13-17
To date, several studies have quantified the dissolving capacity of a solvent by measuring the weight of GP before and after exposure to it, but none sought to test changes in the physical properties.18-20 Such alterations in the material following solvent exposure are important as they can make removal by mechanical instrumentation easier, or, indeed, more difficult. Properties such as hardness and penetrability are particularly important as mechanical files are required to engage the GP in the root canal to allow its removal.
The hardness of a material refers to its ability to resist indentation, which affects the mechanical file's ability to engage the GP in the root canal. Penetrability is difficult if not impossible to measure directly, but deformation energy and resilience can serve as its proxies. Deformation energy is the energy required to deform a material during penetration, whilst resilience refers to the ability of a material to deform while absorbing the energy of the applied load, with subsequent recovery. Thus, a decrease in deformation energy and resilience would ease file penetrability into the GP.
The aim of this study was to use textural analysis to test the changes in the hardness and penetrability of three types of commercial GP when exposed to two types of solvents, and to deduce whether these changes would be of benefit to the operator during endodontic retreatment. Whilst textural analysis features very scarcely, if at all, in the dental literature, it is frequently employed in pharmaceutical research laboratories and the food industry. It plays an invaluable role in determining the properties of materials including, inter alia, rigidity, resilience, cohesiveness, and adhesiveness. Rigidity, deformation energy and resilience were the parameters applicable to this study, as they can be used to represent hardness and penetrability.
METHODS
Sample size calculation
The sample size estimation, performed in G*Power,21 was based on the combined influence of GP type (three types) and solvent type (two types) on each of the outcome variables (hardness and penetration), as determined by a two-factor ANOVA with interaction. Sample size estimations were based on a significance level of 5%, a power of 80% and the effect sizes calculated from pilot data. From these calculations, and considering that each individual test would yield measurements on all three outcome variables, ultimately each group required nine sets of data, requiring a total of 81 experiments.
Materials
The solvents were Xylene BP and Eucalyptus oil BP (Merck South Africa) with distilled water as control. The three different types of GP were: conventional Protaper® size F3 GP cones, thermoplastic GP Thermafil® (Dentsply, York, USA) ISO 030 carrier and cross-linked GuttacoreTM (Dentsply, York, USA) 0.04 size 030. Conventional GP has pure β-phase gutta percha and zinc oxide as its bulk constituents.22 Thermafil® (Dentsply, York, USA) comprises warm α phase GP wrapped around a central polysulfone core.23 GuttacoreTM (Dentsply, York, USA) also has warm α phase GP but wrapped around an internal cross-linked GP core.24
Each GP cone from the respective GP type used was from the same manufacturing batch to eliminate variations in physical properties.
Method
The GP cones were placed in Eppendorf vials, labelled according to their experimental group and numbered from 01 - 81. These numbers were then tabulated on an Excel® spreadsheet and randomly arranged into three experimental batches consisting of 27 vials each. The GP from each group was texturally analyzed using the TA.XT Plus® texture analyzer (Stable Micro Systems, Godalming, UK), which was calibrated for weight, force and distance before each test.
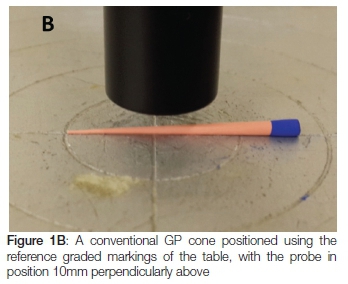
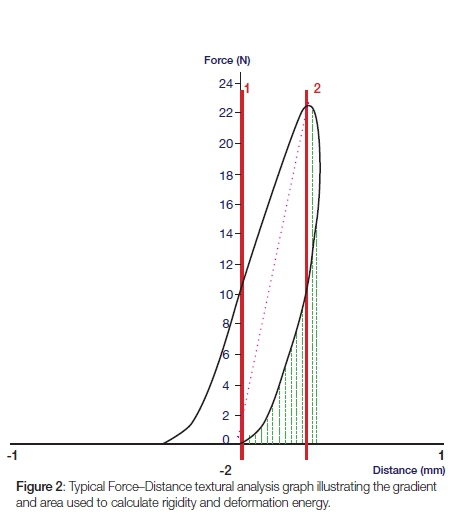
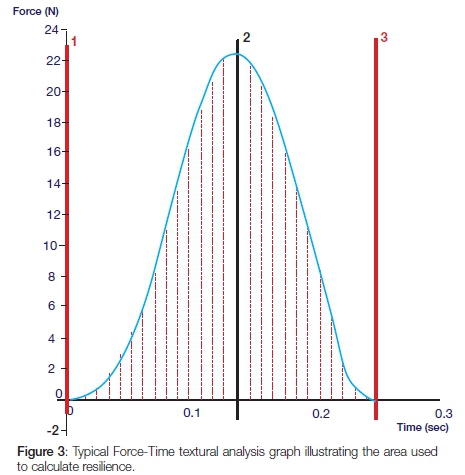
A flat-ended cylindrical probe at a force of 10N with a speed of 5 mm/s-1 was used; each cone was placed against a fixed horizontal platform with graded markings for reproducible positioning of the cones (see Figure 1). The handles of the Thermafil and Guttacore cones prevented the cones sitting flush on the platform and so were removed. After testing prior to solvent exposure, the cones were seated in an Endo stand and box® (Dentsply Maillefer, Switzerland) and immersed into 160 ml of one or other of the solvents at 24±1°C for 10 minutes, followed by immersion in 160 ml of distilled water for 20 minutes to neutralize the solvent action. The cones were allowed to dry for 24 hours at a room temperature of 24±1°C. The ambient room temperature and the temperature of the solvent and distilled water were recorded. The cones were then texturally re-analysed. The software, Exponent®, linked to the equipment, captured data at 200 pps and processed the data into Force-Distance and Force-Time graphs. Rigidity is the gradient of the curve on a Force-Distance graph and deformation energy is the area under the curve on that graph (Figure 2). A Force-Time graph (Figure 3) was used to measure resilience: it is the area from the peak of the curve to the end point divided by the area from the beginning of the curve to its peak, multiplied by 100.
Data analysis
The outcome variable for analysis, for each of the three measurements (rigidity, deformation energy and resilience), was the difference (DIFF) between the post-solvent (AFTER) and pre-solvent (BEFORE) exposure measurements. The use of the DIFF variables was validated by employing randomization to ensure that there were no significant differences in the BEFORE measurements between the three groups within each GP type, assigned to each of the two solvents and the control, water. A General Linear Model (GLM) with main effect for GP type, solvent (nested in GP type), and experiment day as a blocking variable, was used to model each of the BEFORE dependent variables in turn. Post-hoc tests were conducted using the Tukey-Kramer test with the effect sizes calculated using Cohen's d interpreted as follows: >0.80: large effect; 0.50 to 0.79: moderate effect; 0.20 to 0.39: small effect; and <0.20: near zero effect. Between-group differences were assessed by means of a GLM with the outcome variable as the dependent variable; independent variables were the solvent, GP type and solvent-GP type interaction; covariates were room and solvent temperatures.
Comparison of the DIFF results for Xylene and Eucalyptus oil was effected using a one-sample t-test of the DIFF value with respect to 0 to establish whether a significant reduction for a specified parameter was achieved following exposure. A two-sample t-test was performed to determine whether there was a significant difference between the DIFFs of Xylene and Eucalyptus oil. Where the assumptions of these tests were not met, non-parametric alternatives were used, namely the Wilcoxon Signed Rank test and the Wilcoxon Rank sum test. The 5% significance level was employed throughout the study. Data analysis was carried out using SAS software (SAS Institute Inc. USA).
RESULTS
Comparison of GP type before solvent exposure indicated that for Conventional GP, the mean rigidity and deformation energy was significantly lower than in the other two materials (p<0.001), whereas the mean resilience was only significantly lower than that of Guttacore (p=0.021). Comparison of DIFF revealed that the rigidity and deformation energy had either decreased or remained the same following solvent exposure across all groups. Resilience remained unchanged or decreased in all groups except for one, the Conventional GP/Xylene group, where an increase in resilience was observed following solvent exposure.
The comparison of the DIFF results for Xylene and Eucalyptus oil are shown in Table 1.
DISCUSSION
Whilst mechanical instrumentation serves as the primary method for removing GP, chemical solvents assist by softening and partially dissolving the GP in the canal. This study employed textural analysis to assess the changes in the physical properties of three types of GP following exposure to two endodontic solvents. Rigidity, deformation energy and resilience were the parameters applicable to this study, representing hardness and penetrability.
Hardness
The results obtained prior to solvent exposure revealed that Thermafil and Guttacore had higher rigidities than Conventional GP. This can be attributed to the strengthened central cores of the former two types of GP. Distilled water, employed as a control, was incapable of causing any significant reduction in rigidity across all groups.
The rigidities of all the GP types were significantly but variably reduced following exposure to Eucalyptus oil (p<0.05) and Xylene (p<0.05). For Guttacore there was no significant difference between the two solvents. The decrease in rigidity for Conventional GP was significantly greater following exposure to Xylene than to Eucalyptus Oil (p=0.0007; Cohen's d=2.09). In contrast, Eucalyptus oil elicited a significantly greater reduction in rigidity in Thermafil as opposed to Xylene (p=0.019; Cohen's d=1.30).
Conventional GP was less susceptible to a reduction in rigidity, which could be attributed to the comparably thicker quantity of β-phase gutta percha in this GP. Xylene has been shown to weaken the polysulfone core of Thermafil, contributing in this way to a reduction in its rigidity.23 The internal cross-linked core of Guttacore has been shown to resist softening when exposed to solvents.25 These results further support the findings of Mushtaq et al (2012) and Rubino et al (2012) who reported Xylene as an effective solvent of gutta percha.20,26 Magalhães et al (2007) reported that Eucalyptus oil was an acceptable solvent, which differed from previous studies that observed significantly less dissolution efficiency with Eucalyptus oil.9
Penetrability
Deformation energy and resilience serve as representative parameters for penetrability. Any increase in deformation energy following solvent exposure infers that a greater force is required by the retreatment file when penetrating the GP. The deformation energies of all the GP types were significantly reduced with Eucalyptus oil (p<0.05) and Xylene (p<0.05). The decrease in deformation energy for Conventional GP was significantly greater for Xylene than for Eucalyptus Oil (p=0.0006; Cohen's d=2.13). There was no significant difference between the two solvents for Thermafil and Guttacore, but a significantly greater reduction with Thermafil and Guttacore than with Conventional GP (p<0.05). This is in accordance with Tanomaru-Filho et al (2010) who demonstrated that Xylene and Eucalyptus oil presented a greater solvent effect on Thermoplastic GP than on Conventional GP.18
An increase in resilience denotes that the material will absorb a greater energy of the applied force before yielding to penetration or fracture. A decrease in file penetration reduces the surface area of the file that engages the GP. This reduction in contact leads to a decline in the amount of GP being removed with each successive file withdrawal thereby increasing clinical procedure time.
Eucalyptus oil produced no significant reduction in the resilience of Thermafil and Guttacore, with an insignificant increase observed with Conventional GP (p=0.37). Following exposure to Xylene, there was a significant increase in the resilience of Conventional GP (p=0.0175), but a significant decrease with Guttacore (p=0.0015), and an insignificant decrease with Thermafil (p=0.61). Hence, while the solvents may aid the penetration of retreatment files into Thermafil and Guttacore, they may actually confound the retreatment procedure when Conventional GP is present in the canal. Nonetheless, with resilience being closely related to the ability of the polymer chain to rotate freely, additional factors such as the rate and extent of deformation, the applied force, as well as temperature will also affect the resilience value of the material.28 However, increasing the force applied to the retreatment file will amplify the resultant mechanical stresses, which in turn can lead to instrument separation.29
The two crystalline phases (α-phase and β-phase) of GP are molecularly both trans isomers, differing only in single bond configuration and molecular repeat distance. The molecular repeat distance for β-phase GP is shorter than that of α-phase GP. This results in the α-phase being more flexible and contributes to its vulnerability to solvents, as illustrated by Tanomaru-Filho et al in 2010.18 Both Thermafil and Guttacore rely on heating to make their circumferential GP flowable during canal insertion. Heating the material to a temperature range of 46-48°C causes the α-phase GP to transform into β-phase and lose flexibility. Should the heating temperature exceed 58°C, the GP then transforms into an irreversible amorphous phase which then exhibits entirely different mechanical properties.30-32 Thermal treatment was not used in the present study and since thermal exposure causes molecular phase transformations, and changes the bond structure and orientation of the GP, further study is required to assess the changes in the physical properties that may occur. Therefore, the current data may not necessarily reflect the properties of the material at chairside. However, they do provide a base reference for physical changes that occur in GP following solvent exposure.
CONCLUSIONS
Within the limitations of this study, and considering the toxicity profile of Xylene, and the biocompatibility and antimicrobial effects of Eucalyptol, Eucalyptus oil is recommended for use during endodontic retreatment.
Conflict of Interest: None declared.
Acknowledgements
The authors thank the Department of Pharmacy for their assistance with textural analysis, the Faculty of Health Sciences of the University of the Witwatersrand for partial funding, as well as Dentsply (South Africa) for generously donating the required gutta-percha cones. We thank biostatistician Dr P Gaylard for advice and carrying out the statistical analyses.
References
1. Schilder H. Filling root canals in three dimensions. Dent Clin North Am 1967; 11:723-44 [ Links ]
2. Gutmann JL, Kuttler S, Niemczyk SP. Root canal obturation: An update. Academy of General Dentistry. 2010;1-11. http://www.3dfill.com/pdfs/webce090110.pdf. Accessed March 2014. [ Links ]
3. Prakash R, Gopikrishna V, Kandaswamy D. Gutta-Percha: An Untold Story. Endodontology. 2005; 17:32-6. [ Links ]
4. Siqueira JF. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001; 34:1-10. [ Links ]
5. Martos J, Gastal MT, Sommer L, Lund RG, Del Pino FA, Osinaga PW. Dissolving efficacy of organic solvents on root canal sealers. Clin Oral Investig. 2006; 10:50-4. [ Links ]
6. Tasdemir T, Yildirim T, Celik D. Comparative study of removal of current endodontic fillings. J Endod. 2008; 34:326-9. [ Links ]
7. Hülsmann M, Bluhm V. Efficacy, cleaning ability and safety of different rotary NiTi instruments in root canal retreatment. Int Endod J. 2004; 37:468-76. [ Links ]
8. Ezzie E, Fleury A, Solomon E, Spears R, He J. Efficacy of retreatment techniques for a resin-based root canal obturation material J Endod 2006; 32:341-4. [ Links ]
9. Magalhães BS, Johann JE, Lund RG, Martos J, Del Pino FA. Dissolving efficacy of some organic solvents on gutta percha. Braz Oral Res. 2007; 21:303-7. [ Links ]
10. Kaplowitz, GJ. Evaluation of the ability of essential oils to dissolve gutta-percha. J Endod. 1991; 17:448-9. [ Links ]
11. Wilcox LR. Endodontic retreatment with halothane versus chloroform solvent. J Endod 1995; 21:305-7. [ Links ]
12. Ferreira JJ, Rhodes JS, Ford TRP. The efficacy of gutta-percha removal using ProFiles. Int Endod J. 2001; 34:267-74. [ Links ]
13. Azar MR, Khojastehpour LL, Iranpour NN. A comparison of the effectiveness of chloroform in dissolving resilon and gutta-percha. J Dent (Tehran). 2011; 8:19-24. [ Links ]
14. Karlović Z, Anić I, Miletić I, Jukić S, Bošnjak A, Jurić H. Revision of the endodontic filling with solvents eucalyptol, halothane and orange oil. Acta Stomatologica Croatica. 2001; 35:215-9. [ Links ]
15. Oyama KON, Siqueira EL, Santos M. Action of different solvents on the gutta-percha cones. ECLER Endod 1999; 1:1-8. [ Links ]
16. U.S. Department of Health and Human Services, Public Health Service. Fourth Annual Report on Carcinogens, PB 85-134663. U.S Government Printing Office, Washington, DC, 1985 [ Links ]
17. Wourms JD, Campbell AD, Hicks ML, Pelleu GB. Alternative solvents to chloroform for gutta percha removal J Endod. 1990; 16:224-6. [ Links ]
18. Tanomaru-Filho M, Orlando Td, Bortoluzzi EA, Silva GF, Tanomaru JM. Solvent capacity of different substances on gutta-percha and Resilon. Braz Dent J. 2010; 21:46-9. [ Links ]
19. Faria-Júnior NB, Loiola LE, Guerreiro-Tanomaru JM, Berbert FL, Tanomaru-Filho M. Effectiveness of three solvents and two associations of solvents on gutta-percha and resilon. Braz Dent J. 2011; 22:41-4. [ Links ]
20. Mushtaq M, Farooq R, Ibrahim M, Khan FY. Dissolving efficacy of different organic solvents on gutta-percha and resilon root canal obturating materials at different immersion time intervals. J Conserv Dent. 2012; 15:141-5. [ Links ]
21. Buchner A, Erdfelder E, Faul F, Lang A. 2009. G*Power (Version 3.1.2)[Computer program]. http://www.psycho.uniduesseldorf.de/aap/projects/gpower/. Accessed December 2013 [ Links ]
22. Meyer KM, Kollmar F, Schirrmeister JF, Schneider F, Hellwig E. Analysis of shrinkage of different gutta-percha types using optical measurement methods. Schweiz Monatsschr Zahnmed. 2006; 116:356-61. [ Links ]
23. Ibarrola JL, Knowles KI, Ludlow MO. Retrievability of Thermafil plastic cores using organic solvents. J Endod 1993; 19:417-8. [ Links ]
24. Gutmann JL. The future of root canal obturation. Dent Today. 2011; 30:128-31. [ Links ]
25. Beasley RT, Williamson AE, Justman BC, Qian F. Time required to remove Guttacore, Thermafil plus, and thermoplasticized gutta-percha from moderately curved root canals with Protaper files. J Endod 2013; 39:125-8. [ Links ]
26. Rubino GA, Akisue E, Nunes BG, & Gavini G. Solvency capacity of gutta-percha and Resilon using chloroform, eucalyptol, orange oil or xylene; Capacidade de solvência da guta-percha e do Resilon utilizando clorofórmio, eucaliptol, óleo de laranja ou xilol. J Health Sci Inst. 2012; 30:22-5. [ Links ]
27. Görduysus MÖ, Tasman F, Tuncer S, Etikan I. Solubilizing efficiency of different gutta-percha solvents: a comparative study. J Nihon Univ Sch Dent. 1997; 39:133-5. [ Links ]
28. Huson MG, Maxwell JM. The measurement of resilience with a scanning probe microscope. Polym Test 2006; 25:2-11. [ Links ]
29. Bahcall J. Remedying and preventing endodontic rotary nickel-titanium (NiTi) file breakage. Compend Contin Educ Dent. 2013; 34:324-8. [ Links ]
30. Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha: II. The history and molecular chemistry of gutta-percha. Oral Surg Oral Med Oral Pathol. 1974; 37:954-61. [ Links ]
31. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha. V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg Oral Med Oral Pathol 1985; 59:285-96. [ Links ]
32.Maniglia-Ferreira C, Gurgel-Filho ED, Silva Jr JBA, Paula RCMD, Feitosa JPA, Gomes BPFDA, Souza-Filho FJD. Brazilian gutta-percha points. Part II: thermal properties. Braz Oral Res 2007; 21:29-34 [ Links ]
 Correspondence:
Correspondence:
C. Peter Owen
Department of Oral Rehabilitation, School of Oral Health Sciences
Faculty of Health Sciences, University of the Witwatersrand
South Africa,7 York Road, Parktown, 2193
Cell: +27 83 679 2205
Fax: +27 86 553 4800
E-mail: peter.owen@wits.ac.za













