Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.7 Johannesburg Aug. 2016
CLINICAL REVIEW
Oral candidosis: an update on diagnosis, aetiopathogenesis and management
J FourieI; RAG KhammissaII; R BallyramIII; NH WoodIV; J LemmerV; L FellerVI
IBChD., MSc(Odont), MChD(OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIBChD., PDD., MSc(Dent)., MDent (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIIBDS, MDS. Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IVBChD., DipOdont (MFP), MDent (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
VBDS, HDipDent, FCD(SA)OMP, FCMSAae, Hon. FCMSA. Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
VIDMD, MDENT (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
ABSTRACT
Candidosis is the most common oral opportunistic infection and can be caused by any member of the heterogeneous genus Candida. Diagnosis is based on clinical features and on microscopic identification of the candidal hyphae or pseudohyphae on a smear or in a biopsy specimen of the lesional tissue. Although candida in both commensal and pathogenic forms has similar immunogenic properties, commensal yeasts generate a state of immune tolerance while pathogenic hyphae or pseudohyphae provoke an immuno-inflammatory reaction.
The first step in the treatment of oral candidosis is to moderate any local and systemic predisposing factors, and to prescribe a course of topical antifungal agent. Systemic antifungal treatment should be considered only if topical treatment has been unsuccessful or in cases of severe oral candidosis in debilitated or immuno-compromised subjects.
In this paper, we briefly describe the clinical variants, the diagnosis and the management of oral candidosis, and discuss the commonly used pharmacotherapeutic agents.
Key words: oral candidosis, commensal organism, hyphae, nystatin, miconazole, fluconazole, amphotericin B
INTRODUCTION
Candidosis is a common opportunistic oral infection. It is caused by members of the fungal species Candida, most commonly by C.albicans. The tongue, palate and the buccal mucosa are the oral sites most frequently colonised by the fungus.1-4C.albicans is a unicellular dimorphic fungus that can undergo morphogenetic transition from a commensal yeast form to pathogenic filamentous pseudohyphae or hyphae which can invade tissue and cause symptomatic clinical infection.3,5-8 The pathogenic filamentous forms, but not the commensal yeasts, express genes encoding virulent proteins that can facilitate invasion of oral keratinocytes.9
The mere presence of C.albicans in whatever form but without clinical evidence of tissue abnormality cannot be considered to be clinical infection.10,11 Ultimately, the interplay between micro-environmental conditions, systemic and local host factors, and fungal genetic factors will determine whether the candidal micro-organisms will become virulent with the capacity to cause oral candidosis.6,8,11-13
The purpose of this article is to discuss some aspects of the aetiopathogenesis and the management of oral candidosis, focusing on topical antifungal agents.
DIAGNOSIS
Diagnosis of oral candidosis is based on the clinical features of the lesion and on microscopic identification of filamentous fungal elements either in a smear preparation or in a biopsy specimen from the suspected lesion.11,14,15 Although simple and convenient, the sensitivity of the cytological smear tests for erythematous candidosis is low, in contrast to its high sensitivity for pseudomembranous candidosis.6
Although a biopsy is by no means essential for routine diagnosis of oral candidosis, a definitive diagnosis can be made by demonstration of filamentous fungal elements invading the epithelium, with inflammation of the underlying lamina propria. Candida can be seen when stained with periodic acid-Schiff (PAS) or with Gram and Gomori methenamine silver, but not with haematoxylin and eosin.
The various species of candida can be differentiated by the macroscopic characteristics of cultured colonies, by the microscopic morphology of the fungus, by immunohistochemical techniques, and by the polymerase chain reaction technique.6,16,17
CLINICAL SPECTRUM OF ORAL CANDIDOSIS
There are several clinical patterns of oral candidosis (Table 1) but all have similar histopathological features. The superficial layers of the oral epithelium are penetrated by candidal elements with the formation of intraepithelial microabscesses, and with a chronic inflammatory cell infiltrate in the underlying lamina propria.2 In hyperplastic candidosis, the oral epithelium is hyperplastic and acanthotic.18
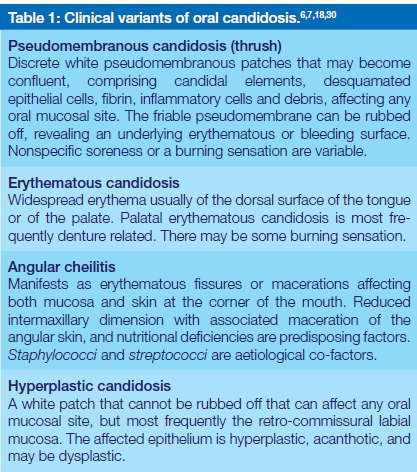
Oral candidosis has been said to occur in acute and chronic forms, and there are those who include median rhomboid glossitis and so-called linear gingival erythema in the spectrum of candida-associated oral lesions.17,19 In agreement with McCullough and Savage,7 the present authors are of the opinion that the terms 'acute' and 'chronic' are redundant and in median rhomboid glossitis, the fungal infection may be adventitious.11
AETIOPATHOGENESIS
The genus Candida comprises about 200 yeast species, with C.albicans accounting for most of candidal infections. However, in recent times, the prevalence of other candidal species in the mouth such as C. glabrata, C. tropicalis, C. krusei, C. dubliniensis and C. parapsilosis appear to have been on the increase as pathogens. It has been reported that candidal species occur as commensals on the normal oral and oro-pharyngeal epithelium of up to 60% of immunocompetent, non-hospitalized subjects who are free of clinically detectable oral candidosis.1,2,16,20
C.albicans exists in the mouth in three different morphological forms: the yeast cell, also termed blastopore or blastoconidium, the septate filamentous form termed the pseudohypha, and the non-septate filamentous form, the hypha.1,2,14 The yeast cell is commensal, avirulent, does not invade the oral epithelium, and in a healthy host, induces regulatory immune responses mediated by keratinocytes and epithelial immunocytes releasing into the local microenvironment a number of biological agents which induce protective immune responses, preventing the development of clinical infection.9,14
The transition from the commensal yeast form to the potentially pathogenic filamentous forms occurs in response to local micro-environmental stress signals on a background of systemic and local predisposing factors (Table 2).2,14 This transition is associated with the production of virulence factors that promote the adhesion of the fungus to the epithelium, colonization, proliferation, and then invasion of the oral epithelium with evasion of protective immune responses by the fungus.14
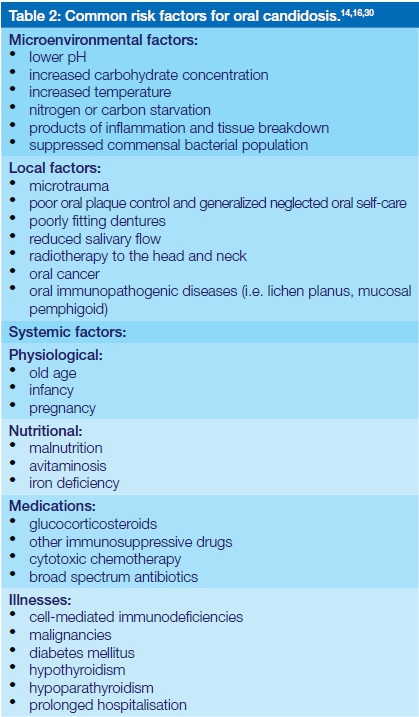
The outcome of this sequence of events is tissue damage with the induction of an immuno-inflammatory response, thus establishing symptomatic clinical infection in the mouth. The effectiveness of the host immune response and the fitness of the invading candidal micro-organism, together with other systemic factors (Table 2) will determine the degree of severity of the infection.14 However, what factors determine the particular presentation of oral candidosis is unknown.18 Nevertheless, it has been suggested that differences in local cell-mediated immunity and variations in the virulence of different strains of C.albicans are important determinants.18
Candidal filamentous elements, but not yeasts, have the capacity to invade the epithelium. Only those species and strains of candida that produce sufficiently potent virulence factors in response to environmental signals will invade the deeper layers of the oral epithelium, causing damage. An inflammatory reaction is induced in the underlying lamina propria, and a clinically apparent oral infection is seen.21-24
Superficial invasion of the oral epithelium by candidal hyphae is seldom sufficient to bring about clinical evidence of inflammation.22,24 The deep invasion of candidal hyphae in the epithelium, however, results in an inflammatory reaction which is protective in nature, and is mediated by Th1 and/or Th17 lymphocytes. Together with their associated cytokines these cells recruit and activate neutrophils and macrophages that are the principle effector cells against the candida, causing the clinically apparent inflammation.14
If invasion of the epithelium with consequent clinical infection is to occur, the candidal hyphae have to penetrate the epithelial layers at a rate faster than the rate of maturation and desquamation of the epithelial cells, otherwise the fungal elements will be shed together with the shedding keratinocytes.14
Oral keratinocytes and dendritic immunocytes are said to have the capacity, through pattern-recognition receptors, to distinguish between the molecular structures of commensal yeast and of filamentous pathogenic forms of candida. The commensal yeasts will activate signalling pathways mediating an overall state of immune tolerance without generating an inflammatory response; but the filamentous forms which become invasive will activate signalling pathways mediating protective immuno-inflammatory responses.8
The transition of candida from a commensal to a pathogenic form causing clinical infection is associated to a great extent with any reduction in the inherent fitness of the immune system. To a lesser extent the transition is influenced by changes in the microenvironment in response to antibiotic treatment, xerostomia, malignancy, systemic chemotherapy, pregnancy, or diabetes with possible secondary reduction in the fitness of the immune system.8,11,19
To add to the complexity of the pathogenesis of oral candidosis, it is now evident that in the mouth, candida usually resides in mixed fungal-bacterial biofilms encapsulated in, and protected by, a matrix of glycoproteins and polysaccharides. Within this biofilm, the bacterial-fungal interactions influence the morphogenetic status, virulence, proliferation and survival of candida.1 Nutrient availability, saliva composition and flow dynamics, and the level of oral cleanliness are some of the local factors that determine the density, thickness and the biological properties of the mixed biofilm.1
TREATMENT OF ORAL CANDIDOSIS
A thorough medical and dental history, and a comprehensive oral examination are essential to identify the presence of predisposing factors for oral candidosis (Table 2). Where possible, such predisposing factors must be eliminated or modified, otherwise the benefits of anti-fungal treatment may be transient.17,19 Regardless of any systemic risk factor, guidance should be provided with regards to improving nutrition and oral health care, cessation of cigarette smoking, and reduction of sugar intake.11,19 Removable dentures of patients with oral candidosis must be well-fitting, thoroughly cleaned both physically and chemically, proper dentogingival plaque control should be effected and the patient be instructed to use an appropriate antimicrobial mouthwash.25,26
Owing to its effective antimicrobial activity against a broad spectrum of micro-organisms, including candida species, chlorhexidine gluconate is frequently used adjunctively in the treatment of oral candidosis, as a twice daily 0.2% mouthwash, and in a 2% solution as an overnight denture disinfectant.27-29
As it is evident that candida proliferates and survives within protective biofilms that are relatively resistant to drug penetration, it is imperative to mechanically disrupt the integrity of the biofilm as soon as oral candidosis is diagnosed. This will interfere with the fungal-supportive ecosystem and will permit the antifungal agent to come into contact with the fungus more readily.30 Therefore, when brushing the teeth, the gingiva, the dorsum of the tongue and the buccal mucosa should also be gently brushed.
Candidal microorganisms are eukaryotic cells having organelles identical to those of human cells. Therefore, there are not many classes of anticandidal pharmacotherapeutic agents that are both effective and non-toxic.19,30 In general, oral candidosis is treated with topical agents which may be either fungicidal or fungistatic. It is preferable to start treatment with topical agents as their side effects and interactions with other drugs are less significant than are those of systemically administered antifungals (Table 3). Frequently used topical antifungal agents include the polyenes, amphotericin B and nystatin, and the azole, miconazole.12,31,32
Polyenes interact with the ergosterol component of the fungal cell membrane, increasing its permeability, allowing leakage of the cytoplasmic content and consequent death of the fungal cell. Polyenes are poorly absorbed by the gastrointestinal tract making them safe for use, with little side effect (Table 3). However the unpleasant taste, and the multiple dosage regimen tend to make for poor patient compliance.27,33-35 Patients who do take these drugs are inclined to quickly swallow or spit them out, before there has been sufficient contact time for therapeutic effectiveness,34 so the patient should be warned about the bad taste, and motivated to use the preparation correctly.
Azoles inhibit the biosynthesis of ergosterol with functional alterations of the fungal cell wall and consequent inhibition of cell multiplication, or cell death.36 Fluconazole and itraconazole given systemically orally are well absorbed in the gastrointestinal tract, and some of the fluconazole is secreted in the saliva giving it an additional topical effect.27 Azoles also inhibit several hepatic cytochrome P450 (CYP) microsomal enzymes, including CYP2C9 which is an enzyme involved in the metabolic breakdown of warfarin, so that the blood concentration of warfarin rises, increasing the potential for bleeding. Even topically applied miconazole oral gel, believed to be only negligibly absorbed in the gastrointestinal track, may result in an increase in the blood concentration of warfarin.37,38
As many other drugs, including diazepam and ritonavir, are metabolized in the liver by the cytochrome P450 enzyme system, the risk of hazardous interactions with azoles, which inhibit the action of the enzyme system, is significant. The prudent clinician should consult the Monthly Index of Medical Specialities Desk Reference (MIMS), before prescribing azoles.37,39 Therefore, nystatin and amphotericin B which are not absorbed in the bowel and thus do not have any significant drug interactions, are to be preferred for the treatment of oral candidosis in patients who are taking other drugs (Table 3).40
As a general rule, any topical agent used in the mouth should be left in situ for as long as possible and accordingly the patient must be instructed not to eat, drink, rinse the mouth or brush the teeth for 40-45 minutes after each dose.41 Any of the topical antifungal agents listed in Table 3 should be equally effective for first line treatment, if correctly used.
If the oral candidosis does not respond to a course of a topical antifungal, the agent should be changed. If treatment is still unsuccessful, the patient should be referred to a specialist in oral medicine for evaluation and for possible treatment with a systemic antifungal agent.6 Systemic antifungal agents used for the treatment of moderate, severe or refractory oral candidosis include fluconazole, itraconazole, posaconazole and ketoconazole. These agents should preferably be given in a liquid form as the local effect, if the patient is instructed to swish-and-swallow, appears to be additionally beneficial. Fluconazole should be used as the first choice systemic antifungal agent.13
SUMMARY
About 60% of healthy subjects have candida in their mouths as commensals. In the great majority of subjects, oral mucosal immunity mediates tolerance of yeast forms, limits colonisation by the potentially pathogenic filamentous forms and generates immuno-inflammatory protection against invasion. Topical antifungal agents are the first line of treatment of oral candidosis. As a general rule, any topical agent used in the mouth should be left in situ for as long as possible, and accordingly the patient must be instructed not to eat, drink, rinse the mouth or brush the teeth for 40-45 minutes after each dose. If topical treatment proves to be ineffective, then a systemic agent should be prescribed.
Conflict of interest: None declared
References
1. Ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res 2009;88:105-15. [ Links ]
2. Feller L, Buskin A, Blignaut E. A review of candida and periodontal disease in immunocompetent and HIV-infected subjects. SADJ 2005;60:152-4. [ Links ]
3. Johnson EM. Rare and emerging Candida species. . Curr Fungal Infect Rep 2009;3:152-9. [ Links ]
4. Touyz L, Harel-Raviv M, Prosterman B, Gornitsky M. Candidal infection of the tongue together with candidal infection of the palate in patients with the human immunodeficiency virus. Quintessence Int 1996;27:89-92. [ Links ]
5. Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000 2009;49:39-59. [ Links ]
6. Farah CS, Lynch N, McCullough MJ. Oral fungal infections: an update for the general practitioner. Aust Dent J 2010;55 Suppl 1:48-54. [ Links ]
7. McCullough MJ, Savage NW. Oral candidosis and the therapeutic use of antifungal agents in dentistry. Aust Dent J 2005;50:S36-9. [ Links ]
8. Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol 1991;45:187-218. [ Links ]
9. Feller L, Altini M, Khammissa RA, Chandran R, Bouckaert M, Lemmer J. Oral mucosal immunity. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:576-83. [ Links ]
10. Borromeo GL, McCullough MJ, Reade PC. Quantitation and morphotyping of Candida albicans from healthy mouths and from mouths affected by erythematous candidosis. J Med Vet Mycol 1992;30:477-80. [ Links ]
11. Manfredi M, Polonelli L, Aguirre-Urizar JM, Carrozzo M, McCullough MJ. Urban legends series: oral candidosis. Oral Dis 2013;19:245-61. [ Links ]
12. Zhang LW, Fu JY, Hua H, Yan ZM. Efficacy and safety of miconazole for oral candidiasis: a systematic review and meta-analysis. Oral Dis 2015. [ Links ]
13. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr., Calandra TF, Edwards JE, Jr., et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503-35. [ Links ]
14. Feller L, Khammissa RA, Chandran R, Altini M, Lemmer J. Oral candidosis in relation to oral immunity. J Oral Pathol Med 2014;43:563-9. [ Links ]
15. Rachanis CC. Looking into the mouth - oral manifestations of HIV infection. SA Fam Pract 2003;45:44-50. [ Links ]
16. Janik MP, Heffernan MP. Yeast infections: Candidiasis and Tinea (Pityriasis) Versicolour. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. New York, USA: McGraw-Hill; 2008. 1822-4. [ Links ]
17. Williams DW, Lewis MA. Isolation and identification of Candida from the oral cavity. Oral Dis 2000;6:3-11. [ Links ]
18. Reichart PA, Samaranayake LP, Philipsen HP. Pathology and clinical correlates in oral candidiasis and its variants: a review. Oral Dis 2000;6:85-91. [ Links ]
19. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol 2011;3: 5771-DOI 10.3402/jom.v310.5771. [ Links ]
20. Patel M, Shackleton JT, Coogan MM. Effect of antifungal treatment on the prevalence of yeasts in HIV-infected subjects. J Med Microbiol 2006;55:1279-84. [ Links ]
21. Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 2010;42:590-8. [ Links ]
22. Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One 2011;6:e17046. [ Links ]
23. Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou A. Invasive phenotype of Candida albicans affects the host pro-inflammatory response to infection. Infect Immun 2005;73:4588-95. [ Links ]
24. Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 2007;75:2126-35. [ Links ]
25. Skupien JA, Valentini F, Boscato N, Pereira-Cenci T. Prevention and treatment of Candida colonization on denture liners: a systematic review. J Prosthet Dent 2013;110:356-62. [ Links ]
26. Koray M, Ak G, Kurklu E, Issever H, Tanyeri H, Kulekci G, et al. Fluconazole and/or hexetidine for management of oral candidiasis associated with denture-induced stomatitis. Oral Dis 2005;11:309-13. [ Links ]
27. Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA. Candida biofilms and oral candidosis: treatment and prevention. Periodontology 2000 2011;55:250-65. [ Links ]
28. Ellepola AN, Samaranayake LP. Adjunctive use of chlorhexidine in oral candidoses: a review. Oral Dis 2001;7:11-7. [ Links ]
29. Barasch A, Safford MM, Dapkute-Marcus I, Fine DH. Efficacy of chlorhexidine gluconate rinse for treatment and prevention of oral candidiasis in HIV-infected children: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:204-7. [ Links ]
30. Rautemaa R, Ramage G. Oral candidosis--clinical challenges of a biofilm disease. Crit Rev Microbiol 2011;37:328-36. [ Links ]
31. Martinez-Beneyto Y, Lopez-Jornet P, Velandrino-Nicolas A, Jornet-Garcia V. Use of antifungal agents for oral candidiasis: results of a national survey. Int J Dent Hyg 2010;8:47-52. [ Links ]
32. Kuriyama T, Williams DW, Bagg J, Coulter WA, Ready D, Lewis MA. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiol Immunol 2005;20:349-53. [ Links ]
33. Anil S, Ellepola AN, Samaranayake LP. Post-antifungal effect of polyene, azole and DNA-analogue agents against oral Candida albicans and Candida tropicalis isolates in HIV disease. J Oral Pathol Med 2001;30:481-8. [ Links ]
34. Garcia-Cuesta C, Sarrion-Perez MG, Bagan JV. Current treatment of oral candidiasis: A literature review. J Clin Exp Dent 2014;6:e576-82. [ Links ]
35. Matsubara VH, Silva EG, Paula CR, Ishikawa KH, Nakamae AE. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis 2012;18:260-4. [ Links ]
36. Katragkou A, Tsikopoulou F, Roilides E, Zaoutis TE. Posaconazole: when and how? The clinician's view. Mycoses 2012;55:110-22. [ Links ]
37. Miki A, Ohtani H, Sawada Y. Warfarin and miconazole oral gel interactions: analysis and therapy recommendations based on clinical data and a pharmacokinetic model. J Clin Pharm Ther 2011;36:642-50. [ Links ]
38. Kovac M, Mitic G, Kovac Z. Miconazole and nystatin used as topical antifungal drugs interact equally strongly with warfarin. J Clin Pharm Ther 2012;37:45-8. [ Links ]
39. Rossiter D. Antifungals for dermatological use. In: Blockman M, editor. South African Medicine Formulary. Cape Town, South Africa: Health and Medical Publishing Group; 2014. 186. [ Links ]
40. Pemberton MN, Oliver RJ, Theaker ED. Miconazole oral gel and drug interactions. Br Dent J 2004;196:529-31. [ Links ]
41. Khammissa RAG, Ballyram R, Wood NH, Lemmer J, Feller L. Glucocorticosteroids in the treatment of immune mediated oral diseases. SADJ 2016;71(2):62-7. [ Links ]
42. Rossiter D. Antimycotics for systemic use. In: Blockman M, editor. South African Medicine Formulary. Cape Town, South Africa: Health and Medical Publishing Group; 2014. 34-7; 184-7; 314-9. [ Links ]
 Correspondence:
Correspondence:
Liviu Feller
Head: Department of Periodontology and Oral Medicine
Sefako Makgatho Health Sciences University
Pretoria
South Africa, 0204
Tel: 012 521 4834
Fax: 012 521 4833
E-mail: liviu.feller@smu.ac.za













