Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.7 Johannesburg Aug. 2016
RESEARCH
In vitro antimicrobial comparison of three commercially available chlorhexidine-based oral rinses
BM AbdalrahmanI; H HolmesII; MT PeckIII; NJ BassonIV
IMSc (UWC), MSc (UMST), BDS (U of K). Department of Periodontology, Faculty of Dentistry, International University of Africa, Khartoum, Sudan
IIMChD, MSc, BChd (UWC). Division of Oral Medicine and Periodontics, University of the Western Cape
IIIDivision of Oral Medicine and Periodontics, University of the Western Cape
IVResearch Department, University of Western Cape Dental Faculty
ABSTRACT
INTRODUCTION: Commercially available chlorhexidine (CHX) formulations differ in their CHX concentrations (0.2% and 0.12%) as well as in various additives including alcohol, antimicrobials such as cetylpyridinium chloride and anti-discolouration chemicals such as ascorbic acid and sodium metabisulphite.
AIMS AND OBJECTIVES: To compare in vitro the antimicrobial efficacies of three different CHX preparations (Corsodyl®, Curasept® and GUM® Paroex®) using 0.2% and 0.12% CHX concentrations as controls.
METHODS: A disk diffusion test was performed using pure cultures of the organisms Streptococcus mutans and Candida albicans, and mixed cultures (facultative and strict anaerobes) prepared from oral rinse samples of 14 study participants. The means and standard deviations of the diameters of inhibition zones were calculated.
RESULTS: A statistically significant difference (p value = 0.0001) was found only in Candida albicans cultures between the mean inhibition zones of the CHX preparation disks. Pure CHX preparations and Corsodyl® showed higher antifungal efficacy than Curasept® and GUM® Paroex.
CONCLUSION: Both CHX preparations (0.12% and 0.2%) and the 0.2% CHX preparation containing alcohol (Corsodyl®) have more potent antifungal properties against C. albicans than alcohol-free 0.12% CHX preparations such as Curasept® and GUM® Paroex®
INTRODUCTION
Chlorhexidine (CHX) is the most commonly used antimicrobial agent in dentistry. Because of its wide range of antimicrobial activity, it has been incorporated as an antiplaque agent into several oral hygiene products such as dentifrices and mouthrinses. Based on its clinical efficacy, CHX is currently regarded as the "gold standard" for evaluating new chemical plaque control agents.1
Most commercially available mouthrinses, including some CHX based mouthrinses, contain alcohol, with concentrations being as high as 14-15%.2 However, the addition of alcohol is controversial because of its potential carcinogenic and tissue irritating properties. Several manufacturers have therefore developed alcohol-free CHX based oral hygiene products as alternatives.2 Limited data exists with regards to the antibacterial efficacy of these products, with studies indicating that alcohol-based CHX formulations are more effective than alcohol-free formulations.2
Commercially available CHX formulations differ in their concentrations as well as in the component additives. Most CHX mouthrinses are prepared in concentrations of 0.2% or 0.12%. In this study, the antimicrobial efficacy of three locally available CHX based mouthrinses i.e. Corsodyl Mouthwash® 0.2% CHX (GlaxoSmithKline, Epping, South Africa), Curasept ADS® 220 Oral rinse 0.2 % CHX (Curaden AG, Krein, Switzerland) and GUM® Paroex® 0.12% CHX (Sunstar Europe S.A., Etoy, Switzerland) were evaluated for their antimicrobial efficacy.
MATERIALS AND METHODS
Study design
An in vitro analytical comparative study was carried out to evaluate three commercially available CHX-based mouthrinses.
Study sample
Oral rinse samples were collected from 14 healthy staff members at the University of the Western Cape Dental Faculty (UWC) who met the inclusion criteria (Table 1).
Specimen preparation and data collection
Each subject was supplied with 10 ml of sterile saline in a universal container and instructed to rinse his/her mouth in the presence of the researcher for 60 seconds and then to return the mouth rinse to the container.6 100µl of the rinse was inoculated onto previously prepared Brain Heart Infusion agar plates (BHI), by spreading the sample over the agar surface with a sterile glass rod. For each oral rinse sample, two plates were prepared, one for facultative anaerobic cultures, and the other for strictly anaerobic cultures. The latter was done to culture Gram negative anaerobic bacteria, such as Veillonella and Fusobacteria.7 The anaerobic conditions were created inside an anaerobic jar utilizing an Oxoid® Gas generating kit (UK), with palladium as a catalyst. A colour indicator was used to signal the transformation to an anaerobic environment. For the facultative anaerobic cultures, an anaerobic incubator was used. The incubation period for both culture types was 24 hours.
Preparation of pure cultures
Pure cultures of S. mutans NCTC 25175 and C. albicans NCTC 36801 were selected, as these microorganisms are known aetiological factors for dental caries and candidiasis respectively. These were cultured in the laboratory for 24 hours. Thereafter, a separate inoculum from each culture was prepared. This was done by selecting an appropriate culture and preparing a suspension thereof in saline using the direct colony suspension method.
The two suspensions (S. mutans and C. albicans) were standardized to 0.5 McFarland standard (corresponding approximately to 1.5 X 108 CFU/ml). The McFarland scale is used for measuring bacterial densities in suspensions. There was no need to standardize the turbidity of the oral rinse samples since its natural turbidity closely approximated that of the 0.5 McFarland standard.
100µl of each suspension was inoculated onto 14 standard BHI plates within a quarter of an hour of the suspension preparation. Sterile glass-rods were used to spread the suspension evenly over the surface of the plate. This produced an acceptable distribution of the bacterial colonies on the surface of the 28 agar plates.
The CHX preparations
Three commercially available mouthrinses, representing the most prevalent CHX-based rinses in South African markets, were purchased from local stores, whilst the controls - (only CHX formulations) were prepared by the Institute of Oral and Dental Research at the Faculty of Dentistry, University of the Western Cape (Table 2).
The control CHX formulations (referred to hereunder as "only CHX") were water based and alcohol free solutions that were prepared in two different concentrations i.e. 0.2% & 0.12%. The only CHX 0.2% acted as a control for Corsodyl® and Curasept ADS® 220 (both containing CHX 0.2% concentration), whilst only CHX 0.12% acted as the control for GUM® Paroex® which contained CHX 0.12%.
Disk Diffusion Test to measure inhibition zones:
The 56 agar plates used for the disk diffusion test were divided equally into four groups as listed below:
1. Group 1: 14 facultative anaerobically cultured plates prepared from oral rinse samples.
2. Group 2: 14 strict anaerobically cultured plates prepared from oral rinse samples.
3. Group 3: 14 plates of pure cultures of S. mutans bacteria.
4. Group 4: 14 plates of pure cultures of the fungus C. albicans.
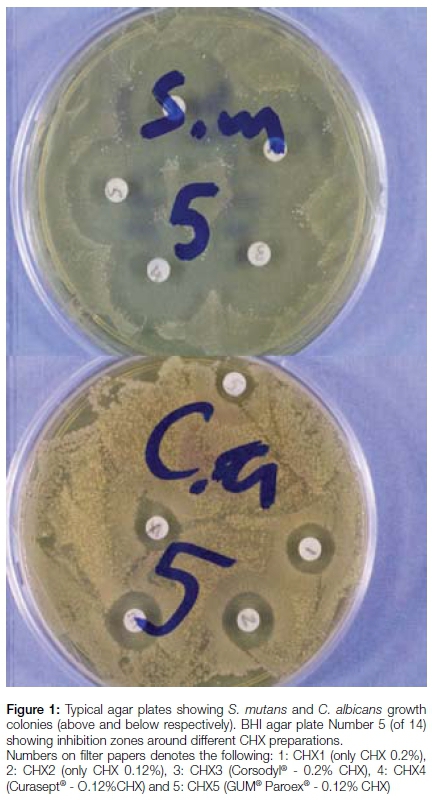
The disk diffusion test was performed by adding five sterile, 6mm diameter filter paper disks to each of the 56 BHI plates. The disks were evenly distributed on the agar surface. Each disk was saturated with 10µl of a specific CHX product to be tested and an identifying code number assigned. The antibacterial effect of each CHX product was quantified in terms of the formation of inhibition zones around the disks, measured 24 hours following incubation (Figure 1).
All measurements were carried out using a digital calliper, by both the principal investigator and by a second clinician, who was blinded to the results obtained by the former. The diameter of each inhibition zone was measured thrice by each investigator. If a discrepancy of >1mm was found, the measurements were repeated. An average of the readings of each investigator was taken. Data capturing tables were used to record the readings.
Data analysis
The mean diameters and standard deviations of the corresponding inhibition zones were calculated and compared using the analysis of variance (ANOVA) test. A P value of less than 0.05% was considered statistically significant.
RESULTS
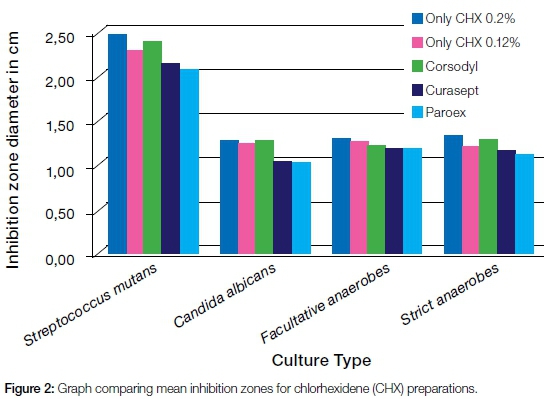
A statistically significant difference in the inhibition zones was found only in the fungal cultures, as analyzed by the ANOVA one-way test. Both Curasept®(0.12% CHX) and GUM® Paroex® (0.12% CHX) showed significantly smaller inhibition zones (p<0.05) while the only CHX (0,2% and 0.12%) as well as Corsodyl® (0,2% CHX), produced inhibition zones of comparable sizes (Table 2). When the inhibition zones produced by the different cultures were compared, all the formulations gave significantly larger zones towards S. mutans than did any of the other cultures. No significant differences were recorded between the zones produced by facultative anaerobic and strict anaerobes from the mixed saliva cultures.
When the means of inhibition zones for all CHX formulations were considered together, readings could be represented as in Figure 2.
DISCUSSION
The results of the study showed no statistically significant differences between the antibacterial activities of the three mouthrinses evaluated (Corsodyl®, Curasept® and GUM® Paroex®), regardless of the CHX concentration or the brand of oral rinse tested. The antimicrobial efficacies of all CHX formulations were highest against Streptococcus mutans, when compared with other cultures, supporting the anti-cariogenic role of CHX and its use as an adjuvant to mechanical oral hygiene measures.8
Even though a statistically significant difference was found with regard to the antifungal activity between the three oral rinses, the inhibition zones produced for C. albicans cultures were smaller than those recorded for S. mutans cultures. Only CHX preparations and Corsodyl® showed a higher antifungal activity compared with Curasept® or GUM® Paroex®. This finding is consistent with the literature where C. albicans displayed a lower susceptibility to CHX. This is thought to be due to the greater complexity of the fungal cell membranes as compared with that of Gram positive bacteria.10
For both S. mutans and C. albicans, the readings for antimicrobial sensitivity across the 14 cultures were numerically closer than in both types of cultures prepared from participants' oral rinses. Such variability reflects the qualitative differences in oral microbial flora between individuals.
Only CHX (0.2%) exhibited the highest antimicrobial efficacy followed by Corsodyl® (containing 5% alcohol) and then Curasept®.The latter has a similar CHX concentration but is alcohol free. Alcohol acts as an emulsifier, as a solvent for active ingredients, as a preservative and as an antiseptic.13 Perhaps the alcohol in Corsodyl® acted as a diluent of CHX and the additive in Curasept® (anti-discolouration systems) could have decreased the antimicrobial efficacy of CHX4, due to interaction with the strong positive charge of the CHX molecule.9
Even though GUM® Paroex® contains 0.05% CPC, it was surpassed in antimicrobial efficacy by pure CHX 0.12%. This could probably also be attributed to the aforementioned chemical interactions between CHX and additives, thereby reducing its efficacy. Nevertheless, GUM® Paroex® had similar antimicrobial efficacy as Curasept® (0.2% CHX), and this was probably due to the fact that it contained 0.05% CPC.
CONCLUSION
The results of the study show that both the pure (0.12% and 0.2%) CHX preparations as well as the 0.2% CHX preparation containing alcohol (Corsodyl®) have more potent antifungal properties against C. albicans than does alcohol-free 0.12% CHX preparations such as Curasept® and GUM® Paroex®. CHX preparations are effective against most classes of oral microflora and is well chosen as an adjunct to plaque removal.
Ethical approval for the study was obtained from the UWC Dental Faculty.
Conflict of Interest: None declared.
Acknowledgements: The authors wish to acknowledge the staff of the Oral Medicine and Periodontology Department for their contribution towards the study.
ACRONYMS
CHX: chlorhexidine
CFU: colony forming units
BHI: Brain Heart Infusion
References
1. Jones, C. Chlorhexidine: is it still the gold standard? Periodontology 2000 1997;15(1):55-62. [ Links ]
2. Herrera, D, Rolda´n, S, Santacruz, I, Santos, S, Masdevall, M., Sanz, M. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: an in vitro contact test and salivary bacterial counts study. Journal of Clinical Periodontology 2003;30:307-14. [ Links ]
3. Sreenivasan, P, Haraszthy, V, Zambon, J. Antimicrobial efficacy of 0.05% cetylpyridinium chloride mouthrinses. Letters in Applied Microbiology 2012;56:14-20. [ Links ]
4. Guggenheim, B, Meier, A. In vitro effect of chlorhexidine mouth rinses on polyspecies. Biofilms' Schweiz Monatsschr Zahnmed 2011;.121:432-41. [ Links ]
5. Arweiler, N. Boehnke, N. Sculean, A. Hellwig, E, & Auschill, T. Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque regrowth study. Journal of Clinical Periodontology 2006, vol.33, pp.334-9. [ Links ]
6. Samaranayake, L, MacFarlane, T. Lamey, P. & Ferguson, M. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. Journal of Oral Pathology 1986;15 (7):386-8. [ Links ]
7. Dzink, J, Tanner, A, Haffajee, A, Socransky, S. Gram negative species associated with active destructive periodontal lesions. Journal of Clinical Periodontology 1985;12(8):648-59. [ Links ]
8. Emilson, C. Susceptibility of various microorganisms to chlorhexidine. Scandinavian Journal of Dental Research 1977; 85:255-65. [ Links ]
9. Gomes, B, Vianna, M, Zaia, A, Almeida, J,. Souza-Filho, F, Ferraz, C. Chlorhexidine in Endodontics. Brazilian Dental Journal 2013;24(2):89-102. [ Links ]
10. Chaffin, W. Candida albicans cell wall proteins. Microbiology and Molecular Biology Reviews 2008;72(3):495-544. [ Links ]
11. Lindhe, J, Karring, T, Lang, N. Clinical Periodontology and Implant Dentistry, 5th edition, Copenhagen: Blackwell Munksgaard, 2008: 187-96. [ Links ]
12. Winn, D, Blot, W, McLaughlin, J, Austin. D, Greenberg, R, Preston- Martin, S. Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Research 1991;51:3044-7. [ Links ]
13. de A. Werner, C, Seymour, R. British Dental Journal 207, E19 (2009) Published online: 28 November 2009 | doi:10.1038/sj.bdj.2009.1014. [ Links ]
 Correspondence:
Correspondence:
Haly Holmes
Division of Oral Medicine and Periodontics, University of the Western
Cape, Oral Health Centre, University of the Western Cape Dental Faculty.
Private Bag X1
Tygerberg; 7505
Tel: 021 937 3102
Cell: 083 231 163
E-mail: hholmes@uwc.ac.za













