Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.71 no.6 Johannesburg Jul. 2016
CASE REPORT
Cancrum Oris (noma) in an HIV-positive adult: A case report and literature review
K PedroI; D A SmitII; J A MorkelIII
IBChD (UWC). Department of Maxillo-Facial- and Oral Surgery, University of the Western Cape, Tygerberg, South Africa
IIBChD (US/UWC), MChD (CommDent)(UWC). Department of Maxillo-Facial- and Oral Surgery, University of the Western Cape, Tygerberg, South Africa
IIIBChD, MBChB, MChD (Stellenbosch). Head (Academic), Department of Maxillo-Facial and Oral Surgery, University of the Western Cape, Tygerberg, South Africa
ABSTRACT
BACKGROUND: Noma refers to an overwhelming invasion of micro-organisms from the oral cavity into the face leading to gangrene, sepsis and, possibly, death. Figures available for 2006 indicate an estimated incidence of 100 000 to 140 000 new cases each year in sub-Saharan Africa with a mortality rate of 70% to 90%.
INTRODUCTION: The classic clinical picture of a noma patient is severe facial tissue destruction associated with oral ulcerations and, in some cases, acute necrotising gingivitis. The following case report concerns a 35-year-old female patient who was treated at the Department of Maxillo-Fa-cial and Oral Surgery at the Tygerberg Oral Health Centre.
CASE REPORT: A 35- year old female was referred to the department with a gaping defect in her right cheek accompanied by necrosis of her mandible. Her medical history indicated that she was HIV+ and had previously been diagnosed with Multi-Drug Resistant TB (MDR). She had defaulted on treatment for both these diseases. Extra-oral examination revealed a gaping defect as a result of the loss of soft tissue of the right cheek. The patient was admitted so that her medical treatment regime could be optimized under supervision.. A biopsy of the exposed mandibular bone and soft tissue was done under local anaesthetic as the patient was considered an anaesthetic risk. The results indicated acanthosis with diffuse epidermal hyperplasia.
DISCUSSION: Globally it appears that HIV infection is not a strong risk factor for noma. In South Africa, HIV infection may play a substantial role in the pathogenesis of noma. In this particular case there was severe facial tissue destruction associated with oral ulceration and acute necrotising gingivitis. An increase in the incidence of noma shows that it cannot be dismissed as a scourge of previous centuries, but remains a public health issue in the poorest communities of the world, still claiming thousands of victims annually. Hence the attempt to reduce the incidence of noma has become a priority for the World Health Organization's (WHO) five-point strategy.
The goal of treatment during the acute stage aims to keep the patient alive by administering antibiotics and specific treatment for co-existing diseases. Once the initial stages have been overcome and a good nutritional status has been achieved, patients can be assessed for reconstructive surgical treatment.
CONCLUSION: The clinical features of this case were consistent with classical features reported in the literature. It emphasizes how this condition contributes towards serious facial destruction and debilitation. This case also highlights a potential association between noma and HIV/AIDS.
BACKGROUND
The word noma is an ancient Greek word that means 'meadow', 'grazing', and, in a metaphorical sense, 'a quickly spreading sore'. In medicine, noma refers to an overwhelming invasion of micro-organisms from the oral cavity into the face leading to gangrene, sepsis and in many cases, death.1
Classical medical authors referred to the condition as Ga-lenus and Celsus. In 1595, Carolus Battus, who settled in the Netherlands, wrote a chapter on 'watercancker' in his Surgeons' Handbook. In 1680 Cornelius van de Voorde introduced the term 'noma' for oro-facial gangrene in chil-dren.1 He was convinced that this condition was not due to a cancerous growth but rather to infection.1
The end of the eighteenth century marks the first successful attempt to reconstruct faces ravaged by noma, while by the mid-nineteenth century medical literature included accounts of extensive facial reconstruction in patients who had survived the disease.1
Noma occurs predominantly among deprived African children living in an impoverished state with low hygiene and inadequate sanitation services.2 Inflammation simultaneously involves the gingival and buccal mucosal surfaces. In 1998, the WHO estimated the annual global incidence of noma at 140 000 and the associated mortality at 70 to 80 percent among patients not treated immediately after diagnosis. A more recent report estimates the annual incidence at 25,000 in developing countries bordering the Sahara.3 Figures available for 2006 indicate an estimated incidence of 100 000 to 140 000 new cases each year in sub-Saharan Africa with a mortality rate of 70 to 90%, i.e. an estimated 75 000 - 100 000 deaths. These figures may be grossly underestimated since less than 15% of acute cases receive medical care.4
Other reasons for uncertain epidemiological data for noma include systematic errors in longitudinal studies, misreporting of cases due to the high mortality rate as well as the stigmatization associated with the disease causing affected children to be shunned by the community and sent to remote locations for medical treatment.5-7
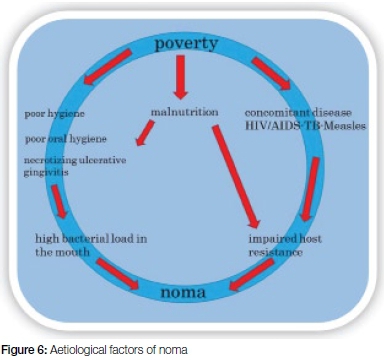
The following are considered host risk factors (etiological factors) associated with noma:
• Malnutrition
• Viral theory (e.g. measles)
• Bacterial theory (e.g. malaria)
• Debilitating diseases (e.g. HIV-AIDS; Tuberculosis (TB); Pneumonia)
Malnutrition
Oral mucosa and the gingiva are characterised by high tissue turnover and can be severely affected by food deficiency, leading to an increased permeability of the tissue membranes. This can facilitate and increase the entry of oral pathogens due to protein and vitamin deficiencies. In addition, the inflammatory response further weakens the tissue membranes. Periodontal tissues and the oral flora are also directly affected by local and systemic consequences of malnutrition. Raised levels of anaerobic flora, gram-negative rods and spirochaetes are present in malnourished children.5,8
Inappropriate weaning practices of various ethnic groups may lead to malnutrition. In some cases breastfeeding is stopped abruptly with no gradual transition to a solid diet. This sudden change in nutrition may lead to deficient levels of vitamins, immunoglobulins, essential aminoacids and minerals. It is at this age that children usually develop kwashiorkor and may also suffer from malaria, measles, other childhood- related diseases and acute noma.5
Viral theory
The viral theory of the aetiology of noma suggests that infection with the herpes virus could lower local immunity, therefore facilitating the development of pathogenic bacterial flora. This hypothesis was originally concerned with the aetiology of acute necrotizing ulcerative gingivitis (ANUG) but has later been extended to include noma.5The global increase in ANUG associated with HIV/AIDS9can also contribute to the increased prevalence of noma. However, the percentage of ANUG or other oral ulcers that transform into noma, is generally very low.3
Tempest believed that measles was an important precursor to noma.10 Measles may lead to lower energy levels and immobilisation of hepatic Vitamin A. If the condition transforms to kwashiorkor or marasmus, it can be fatal. Children also often present with oral ulceration after measles, referred to as noma-like post-measles ulcerations. In addition, tissues impaired by vitamin deficiency are susceptible to the occurrence of noma.10
Micro-organism theory
In less developed countries, the contribution of malaria to noma has been debated. Eckstein postulated that malaria may lead to a decrease in immunity and consequently to noma.11
A malodour arising from the necrosis occurring in the lesions of noma is found in affected patients and has been associated with bacterial infection as an aetiological factor. After overcoming challenging obstacles complicating the investigation of various phases of infection, improved histological techniques have allowed scientists to examine and identify the micro-organisms in the lesions. The main objective of the microbiological analysis is to compare normal and diseased normal flora with a considered emphasis on technique sensitivity.3Prevotella melanino-genica, Corynebacterium pyogenes, Fusobacterium nu-cleatum, Bacteroides fragilis, Bacillus cereus, Prevotella intermedia and Fusobacterium necrophorum have been identified as attendant to noma.
Debilitating diseases
Any debilitating disorder may facilitate the progression of buccal lesions towards noma. Other infectious diseases that have been considered as predisposing factors are chickenpox, smallpox, typhus, typhoid fever, diphtheria, visceral leishmaniasis (kala-azar), pneumonia, TB and, more recently, AIDS.3
The following are social/environmental aetiological factors:
• Poverty
• Poor oral hygiene
• Delay in seeking medical treatment
• Limited access to or inadequate health care facilities
Poverty is an important risk factor, especially in Africa where a lack of resources may lead to poor sanitation, inferior oral and general hygiene and chronic malnutrition. There is also a higher probability of people contracting noma in areas where infective agents are prevalent and communicable diseases are common.3,8,12
In fact, the incidence of noma corresponds to the worldwide geographical distribution of poverty in less developed countries.3 In those countries and definitely in many regions in Africa, the majority of the population do not own a toothbrush. For instance, only a fifth of the children in Nigeria use a toothbrush. Poor oral health can lead to the development of ANUG and research in Nigeria suggested that ANUG can be associated with noma as well as with various other infections.3
INTRODUCTION
The classic clinical picture of a noma patient is severe facial tissue destruction associated with oral ulcerations and, in some cases, acute necrotising gingivitis.3,5,10,13
Given the low incidence of patients presenting in the initial phase, the clinical features of the onset of noma are not as clear. Some of the initial symptoms are fever, malaise, cervical lymphadenopathy, gingival bleeding, oral mucosal lesions, facial oedema and severe halitosis.14
When the oral mucosa and/or overlying skin ulcerate, the destruction can lead to the exposure of underlying bone. It has been suggested that ANUG is a precursor to noma and, hence, the presence of ANUG should raise concern.15The initial phase of the gingivitis commences at the tips of the interdental papillae and marginal gingivae. Blood flow to the infected tissues is inadequate, eventually resulting in an ischaemic area and localised necrosis.16,17 When the necrosis progresses beyond the mucogingival junction, it affects the alveolar, labial, palatal, buccal and lingual mucosa. The full thickness of the buccal or labial muscle layer is now involved and at this point the condition is known as necrotizing stomatitis.18 Further progression will lead to perforation of the facial tissues and skin. In many cases this may eventuate in a matter of days. Generally, the external tissue loss is not closely commensurate with the more extensive intraoral destruction.5 During the separation of the soft tissue slough, sequestration of the exposed teeth and bone occurs spontaneously. In severe cases, larger tissue destruction occurs with the nose, upper lip, infraorbital margin and premaxilla also being affected.5
Possible complications are displacement of teeth associated with tissue destruction and joint displacement, extreme facial deformities, intense scarring, trismus, nasal regurgi-tation, fusion between mandible and maxilla and defective speech. Noma survivors have great difficulty coping with the disfigurement and functional impairment.3,19
As the disease progresses, its systemic effect becomes debilitating. Patients are usually feverish, dehydrated and experience pain.5 Clinical symptoms such as tachycardia and tachypnoea and recurrent diarrhoea may be experienced. It is also common to find a low haemoglobin and white cell count and hypo-albuminaemia. Parasitic infections such as malaria and viruses can be evident.3 Orofacial lesions are characterised by dense scar tissue at the margins of the facial defect with extensive fibrosis.10 These usually occur unilaterally but may also present bilaterally.
The following case report concerns a patient who was treated at the Department of Maxillo-Facial and Oral Surgery at the Tygerberg Oral Health Centre.
CASE REPORT
A 35- year old female was referred to the Department with a gaping defect in her right cheek accompanied by necrosis of her mandible (Figures 1 and 2). Her medical history indicated that she was HIV+ and had previously been diagnosed with Multi-Drug Resistant TB (MDR). She had defaulted on treatment for both these diseases.
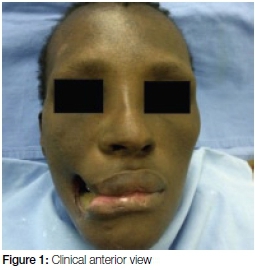
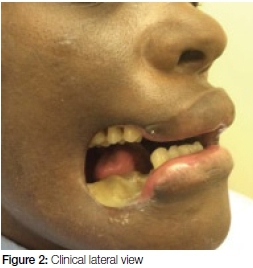
On general examination she appeared cachectic with signs of anaemia, jaundice and she had difficulty walking. Extra-oral examination revealed a gaping defect as a result of the loss of soft tissue of the right cheek, as shown in Figures 1 and 2. The margins of the defect were fibrotic and the exposed mandibular bone appeared necrotic. Intra-oral examination was limited by restriction of opening due to trismus caused by the chronic infection and subsequent fibrosis.
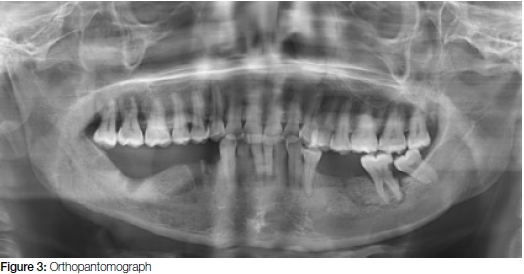
The orthopantomographic radiograph (OPG) (Figure 3) revealed the following:
• bone loss around the 18 and 17 teeth suggestive of periodontitis
• caries and periradicular radiolucencies in the 36 area suggestive of osteomyelitis
• visible extraction sockets (Figure 3) of the 44, 45, 47, 48 teeth with radio-opacity of the bone suggestive of sclerosing osteitis.
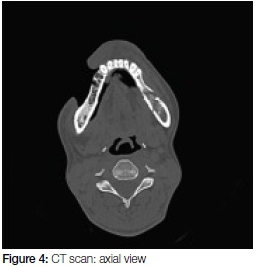
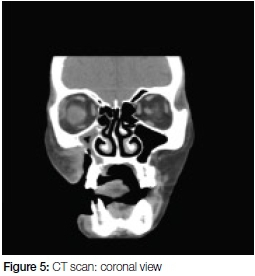
The CT scan (Figures 4 and 5) confirmed the signs of osteomyelitis seen on OPG. Further, the scan showed a large defect (20mm wide) overlying the right maxilla (Figure 5) as well as in the lateral wall of the right maxillary sinus. The sinus was opacified by circumferential mucosal thickening. Inflammatory stranding was noted in the pre-maxilla fat space as well as within the masseteric space.
Special investigation confirmed the HIV+ status and revealed a low CD4 count (15cells/mm3). All liver function indicators (i.e. total bilirubin, conjugated bilirubin, aspar-tate aminotransferase (AST), alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT) were elevated which explained the clinical jaundice. The urea, creatinine and electrolyte test indicated low urea 1.2mmol/L and creatinine 18μmol/L levels which could be attributed to the malnutrition and liver dysfunction. The full blood count confirmed the clinical anaemia with haemoglobin of 6.8g/ dL. The chest x-ray revealed mild signs of post-primary pulmonary TB. The abdominal ultra-sound revealed the following: bilateral hyperechoic kidneys; hyperechoic liver and moderate ascites.
The patient was admitted to optimize her medical treatment regime. She was started on empiric intra-venous amoxicillin and clavulanic acid 1.2g and metronidazole 500mg 8-hourly, commenced XDR TB treatment and received supplementary magnesium-sulphate and calcium.
A biopsy of the exposed mandibular bone and soft tissue was done under local anaesthetic as the patient was considered an anaesthetic risk. This tissue was sent for histology, gene-analysis and fungal culture. The histologi-cal results indicated acanthosis with diffuse epidermal hy-perplasia. No TB was cultured.
Formal sequestrectomies and free vascularised tissue transfers were planned. Regrettably the patient passed away before treatment could be delivered.
DISCUSSION
The epidemiology of noma in the South African population is unknown, and the clinico-pathological features are poorly characterised. Globally it appears that HIV infection is not a strong risk factor for noma. However, in South Africa, HIV infection may play a substantial role in the patho-genesis of noma.18
In this particular case there was severe facial tissue destruction associated with oral ulceration and acute necrotising gingivitis. This is consistent with the classical features of noma (cancrum oris) found in other reported cases.3,5
WHO strategy against noma
An increase in the incidence of noma shows that it cannot be dismissed as a scourge of previous centuries, but remains a public health issue in the poorest communities of the world, still claiming thousands of victims annually. Hence the attempt to reduce the incidence has become a priority for the five-point strategy defined by World Health Organization.1,5
1. Epidemiology and Surveillance
Most noma cases are reported in developing countries in Africa, Asia and South-America. It is estimated that there are about 100 000 new cases of noma yearly, with fatality rates of 80% in the absence of treatment. Due to the challenge of collecting reliable data, the WHO has developed tools for epidemiological studies on referred cases and national retrospective surveys of orofacial mutilations and noma. The WHO also recommends including noma in existing epidemiological surveillance systems.
2. Aetiological research
Research on the aetiology of noma was improved in the 1990's (Figure 6). Poverty, inadequate sanitation, malnutrition, poor oral hygiene, lack of general hygiene and predisposing infectious disease such as HIV/AIDS, concomitant TB and measles are all considered aetio-logical factors.
3. prevention
In its strategy to contain noma, the WHO has made prevention a high priority. Efforts include incorporating an awareness of noma and its symptoms into existing health education and promotional activities together with a call to urgent action to contain the disease, Furthermore, training of primary health care workers in a recognition of the condition to ensure early detection and timeous management could also play a pivotal role in curbing the devastation that noma wreaks in the life of a victim.
4. primary health care
A leaflet describing the progress of noma from necrotizing gingivitis to the loss of tissue was published by the WHO in 1994. The WHO encourages integration of noma detection and management into existing health services, especially at a primary health care level, and recommends that all district personnel be trained in its recognition, management and referral.
5. surgical rehabilitation
Survivors of noma who are left with deformities need plastic and reconstructive surgery. In 2001, the WHO recommended that the Noma Children's Hospital in Nigeria should receive additional resources and designated the Regional Referral Centre for the management of noma cases and the training of personnel from other countries in the region.
TREATMENT OPTIONS AND TIMING OF RECONSTRUCTIVE SURGERY
Differentiation between the acute and late stages of noma is imperative in the determination of the specific treatment option. In the late stage the patient has survived gangrene, sloughing, sequestration, the formation of granulation tissue, wound contracture and re-epithelialization.1
The goal of treatment during the acute stage aims to keep the patient alive by administering antibiotics and specific treatment for co-existing diseases.1 Once the initial stages have been overcome and a good nutritional status has been achieved, patients can be assessed for reconstructive surgical treatment.1,3,5,18,19
CONCLUSION
Cancrum oris remains rare in South Africa, but this case provides evidence that it is still present and that it continues to be a challenge to manage. The clinical features of this case were consistent with classical features reported in the literature. It emphasizes how this condition potentially leads to serious facial destruction and debilitation. This case also highlights a possible association between noma and HIV/AIDS.
Conflict of interest: None declared.
Ethical approval: Informed consent was obtained from the patient.
ACRONYMS
ALP: alkaline phosphatase
ANUG: acute necrotizing ulcerative gingivitis
AST: aminotransferase
GGT: gamma glutamyl transpeptidase
OPG: orthopantomographic radiograph
MDR: Multi-Drug Resistant
XDR: Extreme Drug Resistant
References
1. Bos K, Marck K. The surgical treatment of noma. Netherlands; Uitgeverij Belvédére/Medidact, 2006. [ Links ]
2. Thorpe S. Oral health issues in the African region: Current situation and future perspectives. Journal of Dental Education 2006;70(11):8-15. [ Links ]
3. Enwonwu CO, Falkler WA, Phillips RS. Cancrum oris). The Lancet 2006;368 (9530):147-56. [ Links ]
4. Bouman MA, Marck KW, Griep JEM, Marck RE, Huijing MA, Werker PMN. Early outcome of noma surgery. Journal of Plastic, Reconstructive and Aesthetic Surgery 2010;63 (12):2052-6. Available at: http://dx.doi.org/10.1016/j.bjps.2010.02.012. [ Links ]
5. Baratti-Maye D, Pittet B, Montandon D, Bolivar I, Bornand JE, Hugonnet S, Jaquinet A, Schrenzel J, Pittet D. Noma: an "infectious" disease of unknown aetiology. The Lancet 2003;3(7):419-31. [ Links ]
6. Tonna JE, Lewin MR, Mensh B. A case and review of noma. Public Library of Science Neglected Tropical Diseases 2010;4:869. [ Links ]
7. Adolph HP, Yugueros P, Woods JE. Noma: a review. Annals of Plastic Surgery 1996;37:657-68. [ Links ]
8. Idigbe EO, Enwonwu CO, Falkler WA, Ibrahim MM, Onwuje-kwe D, Afolabi BM, Savage KO, Meeks VI. Living conditions of children at risk for noma: Nigerian Experience. Oral Diseases 999;5:156-62. [ Links ]
9. Vernon LT, Demko CA, Whalen CC, Lederman MM, Toossi Z, Wu M, Han YW, Weinberg A. Characterizing traditionally defined periodontal disease in HIV+ adults. Community Dentistry and Oral Epidemiology 2009;37(5):427-37. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2778275&tool=pmcentrez&rendertype=abstrac [ Links ]
10. Tempest MN.1966. Cancrum oris. British Journal of Surgery 1966;53(11):949-60. Available at: http://www.ncbi.nlm.nih.gov/pubmed/5005023. [ Links ]
11. Eckstein A. Noma. American Journal of Diseases in Children 1940;59(2):219-37. [ Links ]
12. Ogbureke KU, Ogbureke EI. NOMA: A preventable "scourge" of African children. Open Dental Journal 2010;4:201-6. [ Links ]
13. Bolivar I, Whiteson K, Stadelmann B, Baratti-Mayer D, Gizard Y, Mombelli A, Pittet D, Schrenzel J. Bacterial diversity in oral samples of children in Niger with acute noma, acute necrotizing gingivitis, and healthy controls. Public Library of Science Neglected Tropical Diseases 2012;6(3):1556. [ Links ]
14. Lang N, Soskolne W, Greenstein G, Cochran D, Corbet E, Meng HX, Tenenbaum H. Consensus report: necrotizing periodontal diseases. Annals of Periodontology 1999; 4(1):78-87. [ Links ]
15. Jimenez LM, Duque FL, Baer PN, Jimenez SB. Necrotizing ulcerative periodontal diseases in children and young adults in Medellin, Colombia, 1965-2000. Journal of the International Academy of Periodontology 2005;7:55-63. [ Links ]
16. Feller L, Lemmer J. Necrotising gingivitis as it relates to HIV Infection: a review of the literature. Periodontology 2005;2:31-7. [ Links ]
17. Feller L, Lemmer J. Necrotizing periodontal diseases in HIV seropositive subjects: pathogenic mechanisms. Journal of the International Academy of Periodontology 2008;10:10-5. [ Links ]
18. Feller L, Altini M, Chandran R, Khammisa R, Masipa J, Mohamed A, Lemmer J. Review: Noma (cancrum oris) in the South African context. Journal of Oral Pathology and Medicine 2014;43:1-6. [ Links ]
19. Masipa JN, Baloyi AM, Khammissa RAG, Altini M, Lemmer J, Feller. Noma (cancrum oris): a report of a case in a young AIDS patient with a review of the pathogenesis. Head Neck Pathology 2013;188-92. [ Links ]
20. Bourgeois DM, Leclercq MH. The World Health Organization initiative on noma. Oral Diseases 1999;5(2):172-4. [ Links ]
 Correspondence:
Correspondence:
Kim pedro
Tygerberg Oral Health Centre, Private Bag X1, Tygerberg, 7505, South Africa.
Tel: +27 21 937 3119,
E-mail: doc_kpedro@yahoo.com