Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.71 n.2 Johannesburg Mar. 2016
CLINICAL REVIEW
Glucocorticosteroids in the treatment of immune mediated oral diseases
RAG KhammissaI; R BallyramII; NH WoodIII; J LemmerIV; L FellerV
IBChD, PDD, MSc(Dent), MDent (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health sciences University, Pretoria, South Africa
IIBDS, MDS. Department of Periodontology and oral medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIIBChD, DipOdont (MFP), MDent (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IVBDS, HDipDent, FCD(SA)OMP FCMSAae, Hon. FCMSA. Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
VDMD, MDent (OMP). Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
ABSTRACT
Glucocorticosteroids are indispensable agents in the treatment of mucocutaneous immune-mediated diseases because of their anti-inflammatory and immunosuppressive properties. Short-term use of glucocorticosteroids is relatively safe, but long-term use may have serious adverse effects. Prednisone is the glucocorticosteroid most widely used systemically. It is inexpensive, and because of its intermediate duration of activity the risk of suppression of the hypothalamic-pituitary-adrenal axis is reduced.
Abrupt discontinuation of long-term glucocorticosteroid may cause withdrawal symptoms including malaise, low-grade fever and mood alterations, and at times may allow the return of the acute symptoms of the disease. Accordingly, termination of long-term use of glucocorticosteroids should be done gradually by tapering down the dose.
This appraisal discusses core mechanisms of action of glucocorticosteroids and describes the appropriate dosage regimens of the drug in relation to immune mediated oral diseases.
Key words: Prednisone, hypothalamus pituitary adrenal axis, cortisol
INTRODUCTION
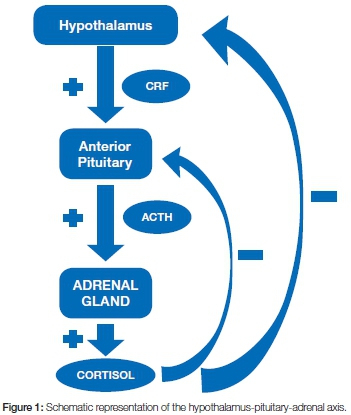
Endogenous cortisol (hydrocortisone), is a glucocorticoid physiologically produced by the adrenal gland cortex. It functions as a regulator of protein, carbohydrate and lipid metabolism, and of inflammatory and immune reactions. The production of cortisol is induced by pituitary adrenocorticotropic hormone (ACTH) which itself is under the positive control of hypothalamic corticotropin-releasing factor (CRF). In turn, cortisol controls the secretion of pituitary ACTH and hypothalamic CRF via negative feedback loops (Figure 1).2 Under basal conditions, the adrenal cortex secretes 20-30mg of cortisol per day in a circadian rhythm, most in the early morning before waking, with secretion diminishing during the course of the day. Under conditions of stress, cortisol production may reach 200-300mg per day.3 Changes in the pattern of sleep may affect the circadian rhythm and hence the cycle of cortisol secretion.2
Ninety percent of cortisol is carried in a protein-bound form in the plasma, most of it bound to α-globulin (transcortin), the rest to albumin. The remaining 10% of plasma cortisol is free and this is the active fraction of the steroid that enters the target cells and exerts the biological effects. The plasma half-life of cortisol is somewhat more than one hour, and the tissue half-life is less than 12 hours (Table 1).2,3
As plasma transcortin is fully occupied by endogenous cortisol, exogenous glucocorticosteroids bind with low-affinity to albumin which can carry substantial amounts, raising the plasma level above the physiological range of glucocorticosteroids.1,3 The resulting negative feedback inhibits secretion of CRF and ACTH with suppression of the hypothalamic-pituitary-adrenal (HPA) axis, which in turn suppresses the physiological activity of the adrenal cortex, making it liable to atrophic changes, with secondary adrenal insufficiency. The degree of such secondary adrenal cortical insufficiency is unpredictable but is related to the dose, duration of use and to the potency of the exogenous glucocorticosteroid, and may persist for more than nine months after cessation of use of the drug.2
Subjects with adrenal cortical insufficiency secondary to the use of medicinal glucocorticosteroids have a reduced capacity to tolerate stress, and should therefore be given supplemental doses of glucocorticosteroids during stressful infectious or surgical events.4 However, recently it has been stated, that for patients at risk of adrenal suppression owing to corticosteroid treatment, and who are undergoing dental or oral surgical procedures under either local or general anaesthesia, corticosteroid supplementation is not necessary.5
Adrenal insufficiency secondary to medicinal glucocorti-costeroids may be minimized by using, whenever possible, a single morning dose of a relatively short-acting agent, or by using an intermediate-acting agent on alternate mornings. With the reduction of the exogenous glucocor-ticosteroids, substantial functional recovery of the adrenal cortex occurs on the day 'off', reducing the risk of development of adrenal cortex insufficiency.3
MECHANISM OF ACTION OF CORTICOSTEROIDS
Glucocorticosteroids enter cells passively and bind to cytoplasmic receptors, forming a receptor-steroid complex which translocates to the nucleus and there interacts with specific DNA sequences, regulating the expression of gene encoding proteins that function as biological modulators. The interaction with transcription factors such as activator protein (AP)-1 and nuclear factor (NF)-kP, downregulates cytokine production and inflammatory processes.1,4,6
Structurally, all glucocorticosteroids share a basic cyclopentenophenanthrene ring. Modifications to this basic steroid ring structure bring about different mineralocorticoid (sodium retention properties), anti-inflammatory or immunosuppressive effects and each modified steroid has a different biological half-life. Hydrocortisone has substantial mineralocorticoid capacity, but the synthetic glucocorticosteroids prednisone and prednisolone exert only a slight sodium retention effect, and methylprednisolone has none at all (Table 1).2
The synthetic cortisone-analogue prednisone is converted to biologically active prednisolone by the enzyme 11β-hydroxysteroid-dehydrogenase-1 (11β-HSDI) in the liver, but also in adipose tissue, brain, fibroblasts, muscles and immune cells.7-9 Any patient with disease of these tissues and in whom this conversion is likely to be impaired should, if treatment is necessary, receive prednisolone rather than prednisone.
Short-term treatment with glucocorticosteroids may arbitrarily be defined as treatment lasting no more than three to four weeks, while long-term treatment lasts over a period longer than four weeks, and may continue for months or years.3 Short-acting glucocorticosteroids are effective for 8-12 hours, intermediate-acting for 24-36 hours and long-acting for more than 48 hours (Table 1),1,2 but the biological activity gradually diminishes as the agent is metabolised. Prednisone is the most commonly used medicinal glucocorticosteroid because it is effective over a wide range of clinical conditions, it is inexpensive, is well tolerated, has minimal mineralocorticoid effect and is suitable for both short- and longer-term treatment.3
ANTI-INFLAMMATORY AND IMMUNOSUPPRESSIVE EFFECTS OF GLUCOCORTICOSTEROIDS
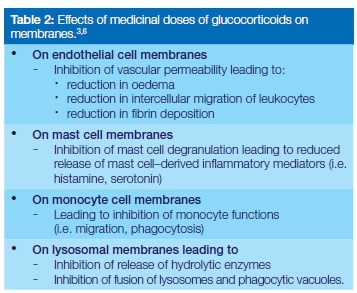
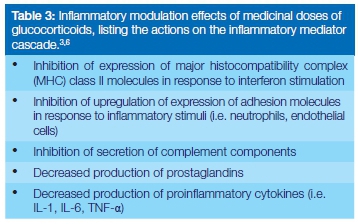
At physiological concentrations, glucocorticosteroids have little anti-inflammatory effect, but at medicinal (supraphysi-ological) concentrations, they affect the functions of biomembranes including those of endothelial cells, mast cells and monocytes, and of lysosomes (Table 2).1,3,4 The production of pro-inflammatory cytokines, inflammatory mediators and other inflammatory factors are also reduced, further suppressing the inflammatory processes (Table 3).1,3,4
Physiologically, glucocorticosteroids have a regulatory effect on the number of lymphocytes in the peripheral blood, but medicinal concentrations of glucocorticosteroids may lead to an accumulation of T lymphocytes and to a lesser extent of B lymphocytes in lymphoid tissue at the expense of the peripheral blood, resulting in a transient lymphopenia.10 The immunosuppressive effects of medicinal glucocorticosteroids are listed in Table 4.

SIDE EFFECTS OF SYSTEMIC MEDICINALGLUCOCORTICOSTEROIDS
The side effects of short-term glucocorticosteroid prescribed for the treatment of oral manifestations of immu-nopathogenic mucocutaneous diseases are spontaneously reversible, but those of long-term administration are more serious, and may be long lasting (Table 5).11,12 Some subjects are at greater risk of the side effects as, for example those with liver disease who do not have the capacity to adequately metabolize the drug, and those with hypoalbuminemia who will have increased levels of free serum glucocorticosteroids.3
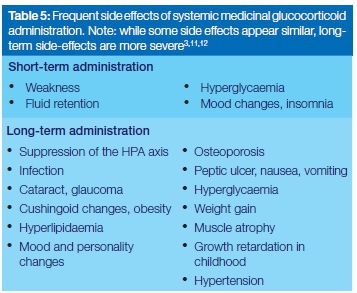
Postmenopausal women, and elderly subjects with decreased physical activity, are inherently vulnerable to osteoporosis, and consequently are at greater risk of developing osteoporosis from medicinal glucocorticosteroids.3 If the step-wise reduction in dosage after long-term use of medicinal glucocorticosteroids is too rapid, some subjects will experience withdrawal symptoms which may include arthralgias, headache, mood changes, lethargy and nausea.3
After discontinuation of long-term treatment with glucocorticosteroids, full recovery of adrenal cortical function may take up to a year. When stressful events, such as surgical procedures, are anticipated, or arise unexpectedly (infections, physical or mental trauma, dehydration), supplemental doses of glucocorticosteroids may be needed.3 However, the severity of suppression of the HPA-axis (Figure 1) varies from case to case and the time required for its functional recovery after discontinuation of the steroid also varies unpredictably.4
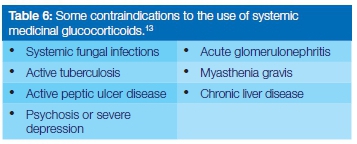
Some contraindications for the use of glucocorticosteroids13 are shown in Table 6. It is noteworthy that HIV infections or even AIDS are not contraindications to the use of shortterm glucocorticosteroids.3
GENERAL PRINCIPLES OF TREATMENT WITH GLUCOCORTICOSTEROIDS
There are no strict evidence-based guidelines for the use of glucocorticosteroids in the treatment of immune mediated blistering, erosive or ulcerative oral diseases. Whether to use topical or systemic agents, and the dosages and durations of the drug regimens to be prescribed, are decisions based largely on expert opinion and on personal experience, with attention to several basic rules.1 Despite the wide range of agents available (Table 7) intermediate acting prednisone remains the systemic drug of choice in oral mucosal diseases, since it has little undesirable mineralocorticoid effect.1,3,4
A wide range of glucocorticosteroids for topical use is available, each having specific degrees of potency, and different durations of activity (Table 8). Most oral mucosal diseases requiring the use of topical application or sub-lesional injections of glucocorticosteroids can be satisfactorily managed with quite a small spectrum of agents, as will be outlined below.
In severe acute cases of immune mediated blistering, desquamative, erosive or ulcerative oral diseases, treatment can start with a dosage of prednisone of 1-1.5mg/ kg/day; and for less severe to milder cases with 40-60-mg per day, according to the judgement of the clinician.1,3 For best clinical results and to maintain both a more uniform serum level and a more consistent therapeutic level, daily amounts of prednisone are given in three doses.3 Each patient receiving orally administered glucocorticosteroids should be provided with a typed schedule, setting out the number of tablets to be taken, morning, noon and evening, to avoid confusion.
It is important to complete any course of systemic gluco-corticosteroids, even at quite low dosages, with a gradual tapering-off to allow the adrenal cortex to recover from the negative feedback effect of the drug on hypothalamic CRF and on pituitary ACTH (Figure 1). There are several ways to manage this tapering-off process, but for the thrice-a-day dosage regimen outlined above, a rule of thumb is that the greater the initial dosage and the longer the course of treatment, the longer and more gradual should be the ta-pering-off process. The lesser midday and evening doses should be reduced first, and then the morning dose.3,14
As the immune-mediated oral diseases, the subject of this article, are nearly always 'managed' rather than cured, and depending on the response to the treatment, the morning dose may sometimes be continued as a maintenance regimen at the lowest level sufficient to contain the disease. This might be anywhere between 2.5mg and 20mg of prednisone, as determined by trial and observation.3,4 After the course of systemic corticosteroid treatment, maintenance can often be with a topical glucocorticoster-oid preparation, or by intermittent sublesional injections with slow-release methylprednisolone.
USE OF GLUCOCORTICOSTEROIDS IN THE MANAGEMENT OF IMMUNE-MEDIATED ORAL DISEASES
A recent literature review suggested that acute cases of oral pemphigus vulgaris should be treated with initially high doses of 80-100 mg/day prednisone; and oral erosive lichen planus with starting doses of 20-30 mg/ day until the acute symptoms subside, and thereafter with tapering-down doses.1 In the experience of the authors, a chronic maintenance dose of 5 to 15 mg/day of prednisone is usually needed for long-term control of oral pemphigus vulgaris. Oral mucosal pemphigoid is often refractory, and a higher long-term dosage of prednisone may be necessary to control the disease.1,15 Subjects with recurrent aphthous stomatitis, erosive lichen planus and mucosal pemphigoid can initially be treated with topical or sublesional corticosteroid injections, and systemic treatment is necessary only if there is no favourable response to local treatment.16-19
Topical glucocorticosteroids are available as creams, ointments, gels, and lotions20 (Table 8). The type of preparation affects the local availability of the active agent and the capacity of the tissue to absorb it, but this must be balanced against how well the preparation will remain at the site of application.21 As with systemic glucocorticosteroids, the general rule is that higher potency glucocorticosteroid preparations should be used for short periods and, once there has been some favourable clinical response, other less-potent glucocorticosteroids should be prescribed for maintenance.21
Severe immunopathogenic oral diseases may be treated with both topical and systemic glucocorticosteroids.22 The very potent agent clobetasol propionate, when correctly applied three to five times a day as a cream or an ointment over two to three weeks, may be as effective as systemic glucocorticosteroids in the treatment of erosive oral lichen planus and oral mucosal pemphigoid.22 However, similar beneficial results can often be obtained with less-potent steroid preparations such as betamethasone diproprionate or betamethasone valerate (Table 8).
Generally, very potent topical glucocorticosteroids should not be used continuously for a period longer than three weeks, and high, medium or low potency topical agents should not be used for longer than three months, to avoid side effects.23 For immune-mediated oral conditions, it is seldom necessary to continue the use of the drug for that long to obtain a satisfactory result, though maintenance doses may have to be continued for longer.
Widespread lesions affecting the mouth or oropharynx may be treated four or five times a day with an aerosol of beclomethasone dipropionate or budesonide, which is usually intended for the treatment of asthma,19 or with disodium betamethasone aqueous solution (0.5mg dissolved in 10ml warm water, four times a day).24 Any topical agent used in the mouth should be left in situ for as long as possible; and accordingly the patient must be instructed not to eat, drink, rinse the mouth or brush the teeth for 40 to 45 minutes after each dose.
Opportunistic oral infection with candida is not an uncommon complication of the use of oral topical glucocorticosteroids and if it occurs, oral candidosis can be controlled with topical antifungal agents.19 Should treatment with a topical glucocorticosteroid continue for more than ten days, it is advisable to treat patients concurrently with a topical antifungal agent of the clinician's choice as a prophylactic measure.22,25 In the experience of the authors, it may then be convenient to apply the topical steroid in the form of a cream or a gel together with a topical fungicidal gel such as miconazole gel, four to five times a day; or to use a commercially available preparation (Table 9). These preparations are primarily intended for dermatological conditions, and if used in the mouth, should be applied after drying the affected mucosa with gauze.
CONCLUDING REMARKS
Glucocorticosteroids are effective agents for the treatment of immune-mediated blistering, ulcerative, erosive or desquamative oral diseases. The relative potency of glucocorticosteroids is determined by their basic structure, and by their affinity for intracellular receptors.
To achieve the goals of treatment and to minimize adverse effects, glucocorticosteroids with the least mineralocorticoid activity and in the smallest effective dose should be used for the shortest duration of time necessarily to resolve the condition. Whenever possible shorter-acting rather than longer-acting glucocorticosteroids should be used. Long-term (more than three to four weeks) use of as little as 10mg of systemic prednisone per day can suppress the HPA-axis, although this is unpredictable and unusual.
Uncommonly, even the use of short-term potent topical glucocorticosteroids or of long-term less potent glucocor-ticosteroids can suppress the HPA-axis. When long-term treatment with glucocorticosteroids is anticipated, the patient must be appropriately informed about the possible side-effects and adverse effects of the treatment, and should be given a steroid warning card to carry at all times. It may be helpful if the patient could be advised regarding the costs involved.
Conflict of interest: No conflict of interest declared
ACRONYMS
AP-1: activator protein
ACTH: adrenocorticotropic hormone
CRF: corticotropin-releasing factor
HPA: hypothalamic-pituitary-adrenal axis
NF: nuclear factor κβ
References
1. Georgakopoulou EA, Scully C. Systemic use of non-biologic corticosteroids in orofacial diseases. Oral Dis. 2014;20:127-35. [ Links ]
2. Detjen PF. Corticosteroids in the treatment of allergic diseases. In: Patterson R, editor. Allergic diseases. Fourth Edition ed. Philadelphia: J.B. Lippincott Company; 1993. 853-90. [ Links ]
3. Jackson S, Gilchrist H, Nesbitt LT, Jr. Update on the dermatologic use of systemic glucocorticosteroids. Dermatol Ther. 2007;20:187-205. [ Links ]
4. Greenberger PA. Asthma. In: Patterson R, Zeiss CR, Grammer LC, Greenberger PA, editors. Allergic Diseases Diagnosis and Managment. Philadelphia, USA: J.B. Lippincott Company; 1993. 689-94. [ Links ]
5. Napenas JJ, Kujan O, Arduino PG, Sukumar S, Galvin S, Baricevic M, et al. World Workshop on Oral Medicine VI: Controversies regarding dental management of medically complex patients: assessment of current recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:207-26. [ Links ]
6. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13. [ Links ]
7. Raza K, Hardy R, Cooper MS. The 11 beta-hydroxysteroid dehydrogenase enzymes-arbiters of the effects of glucocorticoids in synovium and bone. Rheumatology (Oxford). 2010;49:2016-23. [ Links ]
8. Madsbad S, Bjerregaard B, Henriksen JH, Juhl E, Kehlet H. Impaired conversion of prednisone to prednisolone in patients with liver cirrhosis. Gut 1980;21:52-6. [ Links ]
9. Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Butt-gereit F. Glucocorticoids. Best practice. Res Clin Rheumatol. 2011;25:891-900. [ Links ]
10. Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. The kaleidoscope of glucocorticoids effects on immune system. Autoimmun Rev. 2011;10:305-10. [ Links ]
11. Scully C, Flint SR, Bagan JV, Porter SR, Moos KF. Mucosal, cutaneous and mucocutaneous diseases. In: Scully C, Flint SR, Bagan JV, Porter SR, Moos KF, editors. Oral and Maxillofacial Diseases: Informa Healthcare. 2010. 100-32. [ Links ]
12. Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr Res. 2014;34:179-90. [ Links ]
13. Haveles EB. Therapeutic drug classification and corticosteroids. In: Perretta M, editor. Delmar's Dental Drug Reference: Delmar Thomson learning; 2000. 47-50. [ Links ]
14. Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirt-schig G. British Association of Dermatologists' guidelines for the management of bullous pemphigoid 2012. Br J Dermatol. 2012;167:1200-14. [ Links ]
15. Taylor J, McMillan R, Shephard M, Setterfield J, Ahmed R, Carrozzo M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:161-71 e20. [ Links ]
16. Escudier M, Bagan J, Scully C. Number VII Behcet's disease (Adamantiades syndrome). Oral Dis. 2006;12:78-84. [ Links ]
17. Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21. [ Links ]
18. Eisen D, Carrozzo M, Bagan Sebastian JV, Thongprasom K. Number V Oral lichen planus: clinical features and management. Oral Dis. 2005;11:338-49. [ Links ]
19. Bagan J, Lo Muzio L, Scully C. Mucosal disease series. Number III. Mucous membrane pemphigoid. Oral Dis. 2005;11:197-218. [ Links ]
20. Jeske AH. Therapeutic management of common oral lesions. In: A.H. J, editor. Mosby's Dental Drug Reference: Elsevier Mosby; 2012. 6-9. [ Links ]
21. Del Rosso J, Friedlander SF. Corticosteroids: Options in the era of steroid-sparing therapy. J Am Acad Dermatol. 2005;53:S50-8. [ Links ]
22. Gonzalez-Moles MA. The use of topical corticoids in oral pathology. Med Oral Patol Oral Cir Bucal. 2010;15:e827-31. [ Links ]
23. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-40. [ Links ]
24. Carrozzo M, Gandolfo S. The management of oral lichen pla-nus. Oral Dis. 1999;5:196-205. [ Links ]
25. Savage NW, McCullough MJ. Topical corticosteroids in dental practice. Aust Dent J. 2005;50:S40-4. [ Links ]
26. Rossiter D. Corticosteroids for systemic use. In: Blockman M, editor. South African Medicine Formulary. Cape Town, South Africa: Health and Medical Publishing Group; 2014. 263-9. [ Links ]
27. Rossiter D. Corticosteroids, Dermatological Preparations. In: Blockman M, editor. South African Medicine Formulary. Cape Town, South Africa: Health and Medical Publishing Group; 2014. 205-69. [ Links ]
28. Rossiter D. Antifungals for dermatological use. In: Blockman M, editor. South African Medicine Formulary. Cape Town, South Africa: Health and Medical Publishing Group; 2014. 186. [ Links ]
 Correspondence:
Correspondence:
Liviu Feller
Head: Department of Periodontology and Oral Medicine, Sefako Makgatho Health Sciences University
Pretoria, South Africa, 0204
Tel: 012 521 4834, Fax: 012 521 4833
E-mail: liviu.feller@smu.ac.za













