Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.70 n.8 Johannesburg 2015
RESEARCH
Contamination and disinfection of silicone pacifiers: An in vitro study
J MolepoI; M MolaudziII
IPhD. Head of Department, Oral Biological Sciences, School of Oral Health Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMSc. Senior Technician, Department of Oral Biological Sciences, School of Oral Health Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
INTRODUCTION: Whilst in use as comforting devices, the nipples of pacifiers are permanently in contact with normal oral flora and saliva, allowing flourishing bacterial bio-films. Effective disinfection will limit contamination, promote oral health and prevent oral infections in children. Studies on pacifier disinfection in South Africa are not well documented.
AIM: To investigate in vitro disinfection of contaminated pacifiers with alcohol-free oral rinse and microwave.
METHODS: Seventy two silicone pacifiers were divided into two groups of 36, and contaminated with standardized suspensions of Candida albicans and Streptococcus mutans respectively. Each group was subdivided into three and disinfected with alcohol-free oral rinse, microwave and sterile distilled water (control), followed by microbiological analysis. Data was analyzed using Kruskal Wallis Anova test.
RESULTS: Alcohol-free oral rinse removed S. mutans from 42% of pacifiers, whilst microwave removed 33%. Microwave removed C. albicans from 83% of pacifiers, but alcohol-free oral rinse removed only 33%, a difference significant at p<0.05. All pacifiers treated with sterile water retained bacterial contaminationed.
CONCLUSION: Microwave was more effective than alcohol-free oral rinse in eliminating C. albicans from pacifiers. Microwave and alcohol-free oral rinse were equally effective in eliminating S. mutans from pacifiers.
Key words: Candida albicans, disinfection, microwave, oral rinse, pacifier
INTRODUCTION
Pacifiers are commonly used as comforting devices during early childhood.1 During use, pacifier nipples are permanently in contact with oral normal flora and saliva, allowing a flourishing of bacterial biofilms.2 These biofilms are important in the formation of plaque, which may lead to dental caries and periodontal disease in children.3 Several studies have shown that used pacifiers have the ability to retain microorganisms.1,2 The use of pacifiers has been associated with otitis media,4 infection by intestinal parasites5 and yeasts.1 There is also evidence that these microorganisms can interact with the immune system, leading to allergies,6 asthma7 and autoimmune diseases.8
However, pacifiers do have their advantages, including soothing infants and affording protection against Sudden Infant Death Syndrome.9 A recent study showed that microorganisms transferred from mothers sucking pacifiers can reduce the risk of children developing allergies.10
Very few studies have investigated disinfection methods for pacifiers. Nelson et al,11and Chamele et al12investigated the effectiveness of alcohol-containing Colgate® PerioGard® Rinse and microwave in removing S. mutans from silicone pacifiers. In both studies, Periogard was found to be as effective as microwave irradiation in eliminating S. mutans from pacifiers.11,12 Da Silva et al.13showed that microwave was the most effective method when compared with boiling in disinfecting pacifiers.
S. mutans is a major cause of dental caries, and is one of the bacteria causing infectious endocarditis in children with congenital heart disease.14 Early childhood caries (ECC) is one of the most common chronic childhood diseases affecting normal health and well-being. Whilst the overall prevalence of dental caries has reduced worldwide, that of ECC remains high and is currently a WHO concern.15 In a study investigating the presence of S. mutans on latex and silicone pacifiers after use, the bacterium was found to predominate on the silicone pacifiers.13
C. albicans is a fungus that causes oral thrush in infants and toddlers, and has been reported as a contaminant on pacifiers. In a study of infants up to 8 months of age, the use of a pacifier was found to be highly associated with the frequency of yeast infection, and C. albicans was one of the most prevalent species found.1 Comina et al.2investigated the microbial contamination of used silicone and latex pacifiers. 80% were found to be predominantly contaminated with Staphylococcus and Candida species.
Although there is evidence showing that used pacifiers can retain oral microorganisms1,2,5 there has been inadequate emphasis worldwide on disinfection methods and their effectiveness in limiting pacifier contamination, including in South Africa. There is therefore a need to investigate disinfection methods for pacifiers to limit their contamination and to promote oral health and prevent oral infections in children. None of the previous studies have tested the efficacy of alcohol-free GUM® Paroex™ Chlorhexidine Gluconate oral rinse in disinfection of pacifiers. The rinse is used to inhibit buildup of plaque thereby reducing gingivitis.
The purpose of the current study was to investigate the in vitro disinfection of deliberately contaminated silicone pacifiers using the two methods of alcohol-free GUM® Paroex™ Chlorhexidine Gluconate oral rinse and of microwave.
Significance of study
The knowledge gained from this study would inform the South African community of the importance of regularly disinfecting pacifiers, thus preventing infections such as early childhood caries and oral candidiasis.
MATERIALS AND METHODS
Study population and methodology
The study was conducted at the University of the Witwatersrand, Johannesburg, South Africa. Permision was granted to conduct the study by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg (W-CJ-130916-2). Seventy two new silicone pacifiers obtained directly from their packages (Golden Baby, CKT Tek Co. Ltd, New Taipei, Taiwan) were included in the study. Thirty six of these pacifiers were soaked in a standardised inoculum of C. albicans (ATCC 90028) at 150x106 cfu/ml for 5 minutes. The remaining 36 pacifiers were soaked in a standardised inoculum of S. mutants (NCTC 1044) at 150x106cfu/ml for 5 minutes. The contaminated pacifiers were randomly divided into three groups of 12 each. Group 1 was sprayed three times with GUM® Paroex™ Chlorhexidine Gluconate Oral Rinse (Sunstar Americas, Inc, Ontario, Canada), group 2 was placed in the microwave oven (Sharp SA, Midrand, South Africa) at 750 watts for seven minutes and Group 3 was sprayed three times with sterile distilled water. After disinfection, Group 2 samples were allowed to cool, and Group 1 was rinsed with sterile distilled water for two seconds to remove excess oral rinse.
Microbiological evaluation
After disinfection, all the pacifiers were aseptically suspended in 20ml of sterile phosphate buffered saline (PBS) for two minutes and vortexed for microbial cell detachment. From the initial suspension, dilutions of 10-1, 10-2 and 10-3 were prepared in sterile PBS, and 0.1 ml of each dilution was plated on Mitis salivarius Bacitracin agar (MBA) for the recovery of S. mutans and Sabouraud Dextrose agar (SDA) agar for the recovery of C. albicans. The SDA plates were incubated at 37°C for 48 hours, and the MBA plates at 37°C (5% CO2) for 48 hours. On conclusion, the numbers of colonies in each plate representing live organisms were counted.
Data analysis
Data were analysed using Kruskal-Wallis ANOVA. Statistical significance was set at the 5% significance level.
RESULTS
Alcohol-free oral rinse removed S. mutans from 42% of pacifiers, as compared with microwave which removed S. mutans from 33% of pacifiers. Microwave removed C. albicans from 83% of pacifiers, as compared with alcohol-free oral rinse, which removed 33%. Sterile water failed to remove either S. mutans or C. albicans colonies from any of the pacifiers (Figure 1).
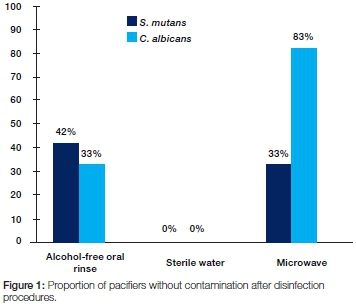
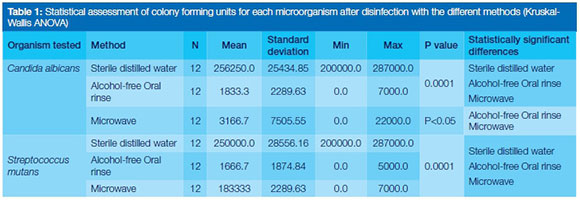
Alcohol-free oral rinse and microwave were statistically similar in eliminating S. mutans (p>0.05). There was a statistically significant difference between alcohol-free oral rinse and microwave in eliminating C. albicans (p<0.05). Statistically significant differences were observed in sterilising efficacy between microwave, alcohol-free oral rinse and sterile water for the removal of both C. albicans and S. mutans (p=0001). (Table 1)
DISCUSSION
Bacterial biofilms flourish during the continuous contact of pacifier nipples with saliva and oral microorganisms.2 Disinfection methods for pacifiers have not been adequately addressed.12 The findings of the current study confirm previous reports that pacifiers can be contaminated by S. mutans .There is a need to investigate effective and easy disinfection methods for pacifiers to reduce micro-bial contamination.
In the current study, alcohol-free oral rinse removed S. mutans from 42% of pacifiers, as compared with microwave which showed a success rate of 33% (not significant at p>0.05). This finding differs from those of previous studies, in which it was shown that Periogard containing an alcohol rinse, and microwave irradiation both eliminated S. mutans from 100% of pacifiers.11,12 The presence of alcohol in the Periogard may have increased the disinfecting effect, as alcohol is a known antiseptic.16 In contrast, a study that investigated the use of mouth rinses with or without alcohol to reduce plaque found both to have similar effects.17 In a previous study,13 S. mutans has been shown to predominate in silicone pacifiers, and microwave removed this organism from 100% of the pacifiers.13
This study revealed that microwave was more effective (83%) than alcohol-free oral rinse (33%) in eliminating C. albicans from silicone pacifiers, showing a statistically significant difference (p<0.05) between the disinfecting methods. Da Silva et al13found microwave to be as effective as boiling water in disinfecting both latex and silicone pacifiers contaminated with C. albicans. Microwave can thus be used for disinfecting silicone pacifiers, as these pacifiers were previously reported to be heat resistant.12 Microwave has also been found to be effective in the disinfection of other dental materials such as prosthesis6, acrylic resins18 and orthodontic instruments.19
S. mutans and C. albicans colonies remained present in all (100%) pacifiers treated with sterile water, confirming that the use of water to clean pacifiers is not effective in eliminating contamination.1,2,11 Chamele et al. demonstrated that S. mutans colonies were still present in 75% of pacifiers treated with sterile water.12
Candida was found by Comina et al. to be one of the most predominant species found in used pacifiers collected from day-care centers.2 Da Silva et al. reported boiling water to be as effective as microwave in removing C. albicans from pacifiers.13
The limitation of the current study is that it was in vitro, carried out on new pacifiers. Different results may be evident if used, worn out pacifiers are assessed in a similar study, for older pacifiers might provide an even more suitable environment for the attachment of microorganisms.
CONCLUSIONS
Based on the sample size and parameters of this study:
1. Silicone pacifiers can be contaminated in vitro with S. mutans and C. albicans.
2. Microwave was more effective than alcohol-free oral rinse in eliminating C. albicans from silicone pacifiers.
3. Microwave and alcohol-free oral rinse were equally effective in eliminating S. mutans from silicone pacifiers.
Conflict of interest: None declared
References
1. Mattos-Graner RO, De Moraes AB, Rontani RM, Birman EG. Relation of oral yeast infection in Brazilian infants and use of a pacifier. J. Dent Child. 2001; 68: 33-6. [ Links ]
2. Comina E, Marion K, Renaud FN, Dore J, Bergeron E, Freney J. Pacifiers: a microbial reservoir. Nurs Health Sci. 2006; 8:216-23. [ Links ]
3. Yonezu T, Yakushiji M. Longitudinal study on influence of pro longed non-nutritive sucking habits on dental caries in Japanese children from 1.5 to 3 years of age. Bull Tokyo Dent Col 2008; 49:59-63. [ Links ]
4. Rovers MM, Numans ME, Langenbach E, Grobbee DE, Ver- heij TJ, Schilder AG. Is pacifier use a risk factor for acute otitis media? A dynamic cohort study. Fam Pract 2008; 25:233-6. [ Links ]
5. Pedroso RS, Siqueira RV. A study on protozoan cysts, helminth eggs, and larvae in pacifiers. J Pediatr 1997; 73:21-5. [ Links ]
6. Rochow EG. Silicon and silicones. Berlin Heidelberg: Spring- er-Velag, 1987. [ Links ]
7. Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol 2006; 117:1133-40. [ Links ]
8. Liu AH. Something old, something new: indoor endotoxin, allergens and asthma. Paediatr Respir Rev 2004; 5 Suppl A: S65-71. [ Links ]
9. Moon RY, Yang DC, Tanabe KO, Young HA, Hauck FR. Pacifier use and SIDS: Evidence for a consistently reduced risk. Matern. Child Health J 2012; 16: 609-14. [ Links ]
10. Hesselmar B, Sjöberg F, Saalman R, Äberg N, Adlerberth I, Wold AE. Pacifier cleaning practices and risk of allergy development. Pediatrics 2013 doi: 10.1542/peds.2012-3345. [ Links ]
11. Nelson-Filho P, Assed S, Raquel S, Luciana S. Disinfection of pacifiers and toothbrushes. J Pediatric Dent. 2011; 33:10-13. [ Links ]
12. Chamele J1, Bhat C, Saraf T et al. Efficacy of microwaves and chlorhexidine for disinfection of pacifiers and toothbrushes: an in vitro study. J Contemp Dent Pract 2012; 13:690-4. [ Links ]
13. Da Silva FC, Spolidorio DMP, Zuanon ACC, Godoi RHM. Pacifier disinfection procedure: superficial morphological aspects and microorganisms colonization. RSBO 2008; 5:30-3. [ Links ]
14. Topcuoglu N, Bozdogan E, Ozsoy SD et al. Prevalence of salivary Streptococcus mutans serotype k in children undergoing congenital heart surgery. J Clin Pediatr Dent 2013; 38:175-8. [ Links ]
15. Peterson PE. Continuous Improvement of Oral Health in the 21st Century - the approach of the WHO Global Oral Health Programme; World Oral Health Report 2003, WHO/NMH/NPH/ORH/03. [ Links ]
16. Morton H E. Alcohols. In: Bloch S S, editor. Disinfection, Sterilization, and Preservation. 3rd ed. Philadelphia, Pa: Lea & Febiger; 1983; 225-39. [ Links ]
17. Leyes BJL, Garcia FM, Gallas TM. Efficacy of chlorhexidine with and without alcohol: a clinical study. J Periodontol 2002; 73:317-21. [ Links ]
18. Mima EG, Pavarina AC, Neppelenbroek KH, Vergani CE, Spolidorio DM, Machado AL. Effect of different exposure times on microwave irradiation on the disinfection of a hard chairside reline resin. J Prosth 2008; 17:312-7. [ Links ]
19. Yezdani A, Mahalakshmi K, Padmavathy K. Orthodontic instrument sterilization with microwave irradiation. J Pharm Bio all Sci 2015;7: Suppl S1:111-5 [ Links ]
 Correspondence:
Correspondence:
J Molepo
Department of Oral Biological Sciences, Faculty of Health Sciences, University of the Witwatersrand
Private Bag 3, Wits 2050, Johannesburg, South Africa
Tel: 011 717 2229. Fax: 011 717 2121
E-mail: Julitha.Molepo@wits.ac.za













