Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.70 no.5 Johannesburg jun. 2015
CASE BOOK
Oral medicine case book 70: Oral Malignant Melanoma
S SinghI; NB ZwaneII; SL ShangaseIII
IBDS, MSc (Wits). Department of Oral Medicine and Periodontology, School of Oral Health Sciences, University of the Witwatersrand
IIBDS (Medunsa). Department of Oral Medicine and Periodontology, School of Oral Health Sciences, University of the Witwatersrand
IIIMDent (Oral Medicine and Periodntology) (Medunsa). Department of Oral Medicine and Periodontology, School of Oral Health Sciences, University of the Witwatersrand
ABSTRACT
Oral mucosal melanoma is an extremely rare, aggressive malignancy that is noticeably distinct from its cutaneous counterpart by its heterogeneity in clinical course, histopathological features and prognosis.
There is limited knowledge on the aetiopathogenesis of oral mucosal melanomas, however, recent studies have been focused towards molecular and genetic studies. Studies on cytogenetic alterations (loss of heterozygosity) and familial patterns of inheritance have been investigated, and these differ genetically from cutaneous melanomas. A possible association between the oral mucosal melanoma and Diabetes Mellitus has been alluded to.
Most clinicians and researchers believe that oral mucosal melanomas arise de novo, and less frequently from a precursor benign melanocytic lesion, unlike their cutaneous counterparts which are believed to arise due to chronic sun exposure. Oral mucosal melanomas may present with a heterogeneous range of macroscopic and microscopic features that can make the clinical and histopathological diagnosis and interpretation difficult.
Key words: Oral malignant melanoma, Invasive malignant melanoma, Oral cancer
INTRODUCTION
Malignant melanomas develop from melanocytes which are derived from the neural crest and are widely distributed throughout all cutaneous, ocular, and mucosal surfaces with the cutaneous types being more common than the mucosal.1 The oral mucosal melanoma (OMM) is an extremely rare, aggressive malignancy that is noticeably distinct from its cutaneous counterpart by its heterogeneity in clinical course, histopathologic features and prognosis.2
There is limited knowledge on the aetiopathogenesis of OMM, however, recent studies have been focused towards molecular and genetic studies. Studies on cytogenetic alterations (loss of heterozygosity) and familial patterns of inheritance have been investigated, and these differ genetically from cutaneous melanomas.3 Unlike their cutaneous counterparts which are believed to arise due to chronic exposure to the sun; most clinicians and researchers believe that OMMs arise de novo, and less frequently from a precursor benign melanocytic lesion.2 Oral mucosal melanomas may present with a heterogeneous range of macroscopic and microscopic features that can make the clinical histopathologic diagnosis and interpretation difficult2 and hence may complicate the therapeutic approach.3 The OMM usually presents at a more aggressive vertical (nodular) growth phase with the underlying submucosa already invaded.4 The aggressive nature of this tumour commonly presents with widespread metastasis in advanced stages with the most common sites of metastases being lymph nodes, liver and lungs. Conventional treatment of oral malignant melanoma includes surgical excision that may be combined with radiotherapy or chemotherapy.3 The five year survival rate has been reported as varying from 0-55%.5
Epidemiology
Oral mucosal melanomas are very rare neoplasms, and account for 1% to 8% of all melanomas in United States and Europe.6 The incidence of this neoplasm and the associated survival rate are very low in Europe, whilst higher incidences have been reported amongst the Japanese,6 African7,8 and North American Indian populations.910 Oral melanomas are usually diagnosed from the 5th to 7th decade of life and carry a poor prognosis because of the vertical growth phase, with frequent metastasis,11 and ample thickness of the tumours4 seen upon first consultation. Studies done on the incidence of oral malignant melanomas in the South African population have been extremely limited with only two cases reported in the past five years.12,13 Early diagnosis and prompt management is essential for improved survival rates and dental clinicians need to be made aware of the different presentations and the need for urgent referral. The tumour usually occurs more in males than females.6,14,15 According to Muller, the male to female ratio is noted at 2.5-3:1.16 Kumar et al also reported a similar ratio,3 but there have been contrasting reports in the literature with some reports suggesting a slightly higher female incidence.17
Diagnosis
The OMM that presents without skin lesions is referred to as the primary oral melanoma (POM). The POM is initially asymptomatic19 and not readily seen. Together with inexperience of surgeons/dentists in its identification during early stages, these factors lead to delayed diagnosis and hence the poor prognosis of mucosal melanomas.2,17 The palate is the most common site of involvement (32%-40%), followed by the maxillary gingiva (16%).4,6,11,19 Other sites that may be involved include the mandibular gingiva (7%), buccal mucosa (7%), lips (7%) and alveolar gingiva (5%)6; all of which are easy to access during routine intraoral clinical examination.3
In cases where POM is suspected, and having no previous history of melanomas, a whole body skin examination and ophthalmic examination must be done to exclude primary cutaneous or ocular melanomas.21 Depending on the size and site of the lesion, an incisional/ excisional biopsy is mandatory when suspicion for oral melanoma is high.19
Lesions which mimic malignant melanomas and should be considered i n differential diagnosis i nclude: amalgam tattoo, Kaposi's sarcoma, vascular blood-related pigments, oral melanotic macule, physiologic pigmentation, drug-induced pigmentation and zygomycosis, the latter particularly in cases having a medical history of diabetes.19
The POM case we are reporting in this paper is of interest to the dental community because of its striking clinical resemblance to an intra-oral zygomycosis infection, which is often associated with acidosis as a result of diabetes mellitus (DM).18
Greene et al proposed three criteria for the diagnosis of primary oral malignant melanoma:22
• The demonstration of malignant melanoma in the oral mucosa
• The presence of e so-called junctional activity' (i.e. melanocytes arranged along the basal layer of the surface epithelium) in the lesion
• The inability to show malignant melanoma at any other primary site
These criteria have been used for many years in the diagnosis of primary oral malignant melanomas. More recent and improved methods are now also utilized together with conventional microscopy. Included are cytogenetic and molecular analyses and in particular the use of sensitive markers such as HMB-45, S-100 protein and NKI/C3, HLA-DR, PCNA and cytokeratin (AE1/AE3).6 About 10% of cases have been reported to be amelanotic, which complicates the diagnosis even further. However more than 95% of lesions are anti S-100 antigen positive and more specific markers including HMB-45, Melan-A and antityrosinase can be used to facilitate the diagnostic process.19
Classification
A simple classification of malignant tumours based on a clinical staging system (primary tumour (T); lymph nodes (N); distant metastases (M): TNM) has been used on oral mucosal melanomas and recognizes three stages (Table 1). This system has been shown to be of prognostic value and is used to direct treatment.19 Current conventional parameters such as Clark's level and the Breslow tumour thickness,2,4,17 are important prognostic indicators for cutaneous melanoma but these were not originally established for mucosal melanomas; and there has been very little evidence to support their direct application to cases of oral malignant melanomas.3
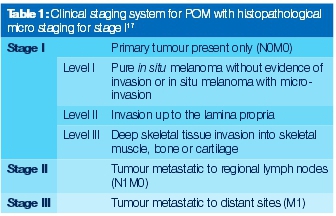
The classification of oral melanomas is very controversial and not well documented compared with the skin melanomas, because of their different behaviour.19 A nomenclature was proposed at the 1995 WESTOP Banff Workshop to classify oral melanomas by histological patterns such as in situ, invasive and, combined invasive and in situ.4,6,17 Since then, researchers have made various attempts at classification systems but none have been confirmed in the literature to date.
CASE REPORT
A 76 year old female patient presented to the Oral Medicine clinic with a complaint of a large 'black growth' on her palate with spontaneous bleeding from the site. The patient only became aware of the 'growth' following the extraction of tooth 26; which had occurred one month prior to consulting with us. The reason for the extraction of tooth 26, as reported by the patient, was increased mobility which made it difficult to chew. Following extraction at the local clinic, she was given antibiotics and subsequently referred to the Oral Medicine Clinic for further investigations, as there was no resolution with antibiotic treatment. At the time of initial examination the patient confirmed that she was receiving treatment for diabetes, hypertension and arthritis.
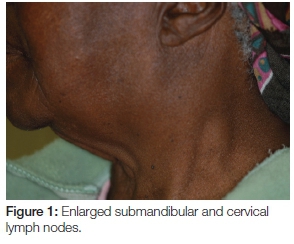
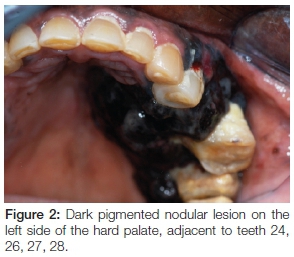
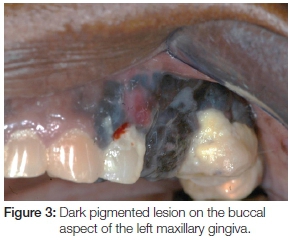
Extra-oral examination discovered enlarged left cervical and submandibular lymph nodes (Figure 1) which were also tender on palpation. Intra-oral assessment revealed a large black-brown fungating mass in the 24-28 region covering almost the entire left side of the hard and soft palate (Figure 2). The palatal lesion was continuous with the lesion on the buccal gingiva via the interdental papillae (Figure 3). Whilst the palatal lesion was homogenous in colour, the gingival lesion was of mixed hue with an erythematous area amid the predominantly brown-black lesion. The patient also presented with poor oral hygiene and severe halitosis. Physiologic pigmentation was noted on the buccal maxillary and mandibular gingiva; and in the 24 area the lesional tissue appeared to be confluent with the physiologic pigmentation.
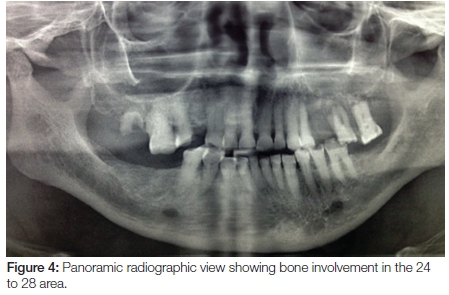
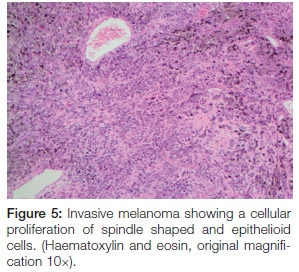
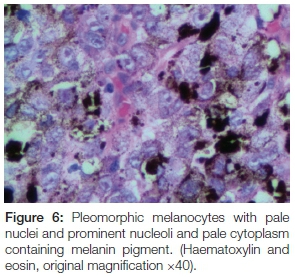
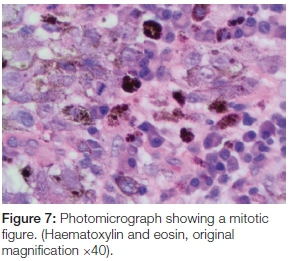
The panoramic radiograph exhibited a 'moth-eaten' radi-olucency in the region of 24-28 with severe bone loss and a loss of lamina dura around teeth 26 and 27 (Figure 4). An incisional biopsy was taken from both the palatal and gingival lesions and the specimens were submitted to the National Health Laboratory Services (NHLS) for analysis. Since the patient had no cutaneous lesions the possibility of a metastatic malignant melanoma to the oral cavity was ruled out in this case. Histologically the tumour showed a cellular proliferation of spindle cells and epitheloid cells (Figure 5). The melanocytes appeared pleomorphic with pale nuclei, prominent nucleoli, brisk mitotic activity and pale cytoplasm containing melanin pigment (Figures 6 & 7). These histological features were morphologically in keeping with an invasive malignant melanoma.
The patient received counselling when the results were made known to her. She was subsequently referred to the Department of ENT for further management. Though there were no distant metastases noted, there was N3 nodal involvement. The patient was placed on the surgical list for resection and soft tissue reconstruction. Unfortunately the patient passed away three months later before undergoing surgical intervention.
DISCUSSION
The primary mucosal malignant melanoma is an exceedingly rare and biologically aggressive tumour.11,19 It is known to account for 0.5% of all malignant oral neoplasms.11 To date, most reported cases of oral malignant melanomas have been associated with an extremely poor prognosis, diagnosis having been at a late-stage of the disease. The reasons for this are not completely understood.3 Garzino-Demo stated that the possible reason for the poor prognosis is not only late detection but the problem could also be related to the histotype, difficulty with the wide resections necessitated during surgery and close proximity to vital structures, early haematogenous metastases and the age of the patient.14
Risk factors for oral mucosal melanoma have not been confirmed, as the oral mucosa, unlike the skin, is not exposed to the sun. Cigarette smoking was considered as a risk factor because of the higher incidence of oral pigmented lesions amongst smokers but studies have not been conclusive.21 Diabetes has been hypothesized as a potential risk factor for melanoma, based on both population-based and clinical surveys which showed a predilection of melanoma to occur in individuals with diabetes.23 The results of this study by Qi et al point to the possibility of diabetes being an independent risk for melanoma, a likelihood further strengthened by the reported increased efficacy of anti-melanotic drugs when used in combination with the anti-diabetic drugs than when used alone.24 The patient presented in this paper was diabetic; and hence the question to be asked is: could her diabetic status have played a role in the development of the melanoma or impacted on its aggression? A number of possible mechanisms through which DM can impact on melanoma have been postulated.23
The complex aetiopathogenesis and the rarity of melanoma have lead to familial risk recently becoming a strong field for investigation by researchers. Future studies are directed at biomarker identification and early detection with the possibility of prevention through the use of predictive genetic testing for melanoma, which is aimed at reducing mortality. However, studies are still relatively new and are mainly focused on the cutaneous forms of melanoma. Whilst oral melanoma develops de novo, it has been linked to precursor non-threatening benign oral melanocytic lesions.2,4 The case that we have reported on had racial/ physiologic pigmentation. The apparent confluence of the lesion on the buccal gingiva of tooth 24 (Figures 2 & 3) with the racial pigmentation can only be suggestive. Though it cannot be suggested that all benign melanocytic lesion will transform to malignancy, nevertheless, given the common link to familial heredity between racial pigmentation and oral melanoma,; it appears prudent to recommend close follow-ups on patients with oral melanocytic lesions, in particular racial pigmentation. Whilst no melanocytic lesion has been confirmed as a precursor lesion for oral melanoma, a third of the patients diagnosed with melanoma have a history of a pre-existing pigmentation at the site of the tumour;4 and when biopsied such pigmentations often display atypical melanocytic hyperplasia.15,25 Close follow-ups are recommended on patients in the age groups between the 5th and 7th decade with melanocytic lesions, particularly physiologic pigmentation. Studies to further explore this association should be encouraged.
Surgery remains the main treatment option. After surgical management and ablation, recurrence and metastasis are frequent events in these cases. Surgery and chemotherapy with or without immunotherapy or radiation therapy is generally the most preferred choice of treatment. Malignant melanoma is generally resistant to radiation therapy but this can be used as an adjunctive treatment when adequate margins are not achieved after surgery due to close proximity to vital structures. Chemotherapy can be used to reduce the size of the tumour for improving surgical management.6 In recent literature surgical excision with a course of IL-2 as adjunctive therapy has been proposed to reduce the incidence of recurrence, but the data regarding this option is still lacking.19
CONCLUSION
The importance of maintaining a high index of suspicion during routine oral examinations by clinicians can never be stressed enough, particularly when pigmented lesions are seen, occurring on the palate and the maxillary gingiva, which are high-risk sites for malignant melanoma. Equally important is the education of patients especially those perceived to be at risk, for them to do regular self-assessment of the oral mucosa and to keep regular review appointments with the clinician, as this may help with early diagnosis of OMM. Clearly not enough is known about melanomas and specifically oral melanomas. However, given their aggressive nature and poor prognosis, research on risk factors and management of oral melanoma is essential to enhance the survival rates of these patients; and should be encouraged.
Declaration: No conflict of interest declared.
ACRONYMS
OMM: Oral Mucosal Melanoma
POM: Primary Oral Melanoma
TNM: primary tumour (T); lymph nodes (N); distant metastases (M)
NHLS: National Health Laboratory Services
DM: Diabetes Mellitus
References
1. Tas F, Keskin S. Mucosal melanoma in the head and neck region: different clinical features and same outcome to cutaneous melanoma. ISRN Dermatol 2013, Article ID 586915, 5 pages, 2013, doi:10.1155/2013/586915. [ Links ]
2. Seetharamu N, Ott PA, Palvick AC. Mucosal melanomas: a case-based review of literature. The Oncologist 2010; 15: 772-81. [ Links ]
3. Kumar SKS, Shuler CF, Sedghizadeh PP, Kalmar JR. Oral mucosal melanoma with unusual clinicopathologic features. J Cutan Pathol 2008; 35: 392-7. [ Links ]
4. Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncology 2000; 36:152 - 69. [ Links ]
5. Gondivkar SM, Indurkar A, Degwekar S, Bowate R. Primary oral malignant melanoma - A case report and review of literature. Quintessence International 2009; 40: 41-6. [ Links ]
6. Gu GM, Epstein JB, Morton TH. Intraoral melanoma: Long-term follow-up and implication for dental clinicians. A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 404 - 13. [ Links ]
7. Broomhall C. Malignant melanoma of the oral cavity in Ugandan Africans. Br J Surg 1967; 54: 581 -4. [ Links ]
8. Snow GB, van der Waal I. Mucosal melanomas of the head and neck. Otolaryngol Clin North Am 1986; 19:537 - 47. [ Links ]
9. Soman CS, Sirsat MV. Primary malignant melanoma of the oral cavity in Indians. Otolaryngol Clin North Am 1974; 38: 426 -34. [ Links ]
10. Black WC, Wiggins C. Melanoma among south-western American Indians. Cancer 1985; 55: 2899 -902. [ Links ]
11. Sortino-Rachou AM, Cancela MC, Voti L, Curado MP. Primary oral melanoma: Population-based incidence. Oral Oncology 2009; 45: 254 -8. [ Links ]
12. Tlholoe MM, Khammissa RAG, Bouckaert M, Altini M, Lemmer J, Feller L. Oral mucosal melanoma: Some pathological considerations and an illustrative report of a case. Head and Neck Pathol. 2015; 9:127 -34. [ Links ]
13. Mosalleum E, Afrogheh A, Dreyer WP, Scheider JW. Oral malignant melanoma. SADJ 2014: 69; 276-8 [ Links ]
14. Garzino-Demo P, Fasolis M, Maggiore GMLT, Pagano M, Berrone S. Oral mucosal melanoma: a series of case reports. Journal of Craniotomy-Maxillofacial Surgery 2004; 32: 251 -57. [ Links ]
15. Barker BF, Carpenter WM, Daniels TE et al. Oral mucosal melanomas: the WESTOP Baniff workshop proceedings. Western Society of Teachers of Oral Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 83: 672-9. [ Links ]
16. Muller S. Melanin-associated pigmented lesions of the oral mucosa: presentation, differential diagnosis and treatment. Dermatologic Therapy 2010; 23: 220-9. [ Links ]
17. Umeda M, Komatsubara H, Shibuya Y, Yokoo S, Komori T. Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncology 2002; 38: 714 -22. [ Links ]
18. Samaranayake LP, Leung KL, Jin L. Oral mucosal fungal infections. Perio 2000 2009; 49: 39-59. [ Links ]
19. Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, Cirillo N. Oral malignant melanoma: a review of the literature. J Oral Pathol Med 2008; 37: 383-8. [ Links ]
20. Takagi M, Ishikawa G, Mori W. Primary malignant melanoma of the oral cavity in Japan, with special reference to mucosal melanosis. Cancer 1974; 34:358-70. [ Links ]
21. Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Pathol 2012; 5: 739-53. [ Links ]
22. Greene GW, Haynes JW, Dozier M, Blumberg JM, Bernier JL. Primary malignant melanoma of the oral mucosa. Oral Surg Oral Med Oral Pathol 1953; 6: 1435-43. [ Links ]
23. Qi L, Qi X, Xiong H et al. Type 2 Diabetes Mellitus and Risk of Malignant Melanoma: A systematic review and meta-analysis of cohort studies. Iranian J Publ Health 2014; 43: 857 -66. [ Links ]
24. Azvolinsky A. Diabetes plus anticancer drug combination targets melanoma cells unresponsive to standard treatments. www.cancercommons.org/2013/06/21, accessed 10/06/2015. [ Links ]
25. Meleti M, Vescovi P, Mooi WJ, van der Waal I. Pigmented lesions of the oral mucosa and perioral tissues: a flowchart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105: 606 - 16 [ Links ]
 Correspondence:
Correspondence:
SL Shangase
Head Department of Oral Medicine and Periodontology
School of Oral Health Sciences
University of the Witwatersrand
Tel: 011 488 4887
Fax: 011 488 4902
E-mail: Sindisiwe.Shangase@wits.ac.za













