Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.70 no.4 Johannesburg Mai. 2015
CASE REPORT
Abnormality in the right mandibular angle and large swelling in the left facial area - an unusual case
Η SabirI; S KumbhareII; P RoutIII; R KumarIV
IMDS (Oral Medicine & Radiology). Lecturer, Dept. of Oral Medicine & Radiology, Government Dental College & Hospital, Nagpur (M.S), India
IIMDS (Oral Medicine & Radiology). Assoc. Prof. and Head, Dept. of Oral Medicine & Radiology, Government Dental College & Hospital, Nagpur (M.S), India
IIIMDS (Oral Medicine & Radiology). Lecturer, Dept. of Oral Medicine & Radiology, Kalinga Institute of Dental Sciences, Bhubaneswar, Odisha, India
IVMDS (Periodontics and implantology). Reader, Dept. of Periodontics, Sri Aurobindo College of Dentistry (M.P), India
ABSTRACT
A large swelling in the facial region of long duration generally suggests benign etiology. We report a case of large asymptomatic swelling in the left facial region which was clinically diagnosed to be a benign salivary gland tumour. The radiological investigations also suggested parotid gland tumour of benign etiology along with a fortuitous discovery of a Stafne bone defect in the right angle of mandible. The histopathological report however confirmed the tumour to be a case of acinic cell carcinoma of the left parotid gland. The patient underwent total parotidectomy and postoperative radiotherapy and has been disease- free for the past three years without any evidence of residual or recurrent lesion. The clinical, radiological, histopathological, and therapeutic aspects of acinic cell carcinoma and the radiological evaluation of the Stafne bone defect are analyzed along with literature review.
Keywords: acinic cell carcinoma; Stafne bone defect; CT; MRI
INTRODUCTION
Acinic cell carcinoma (ACC) is a rare malignant epithelial neoplasm of the salivary glands.1 It accounts for 1 to 6% of all salivary gland tumours and 15% of all malignant tumours in the parotid glands,2 which are the most commonly affected salivary glands (81% to 98%).3 A slowly enlarging mass in the parotid region is the typical clinical presentation.4 It was initially thought to be a benign disease entity until Foote and Frazell in the early 1950s first described it as a carcinoma in recognition of its properties of recurrence and of metastasis.5
Stafne's bone defect was first described by Edward Stafne in 1942.6 It most often appears as a unilateral, ovoid, radiolucent, corticated defect near the angle of the mandible below the inferior alveolar canal and is usually an incidental radiologic finding.7 The bony defect may contain salivary gland, lymphoid tissue, fat, connective tissue, muscle or blood vessels. Empty cavities have also been reported.8 The Stafne bone defect has a prevalence range from 0.1% to 0.48%, with a predilection for men between the age of 50 and 70 years.9
This paper reports a case of ACC of left parotid gland that appeared as a large asymptomatic mass on the left facial region along with a coincidentally-discovered Stafne bone defect in the right angle region of mandible. The clinical, radiological, histopathological, and therapeutic aspects of acinic cell carcinoma along with radiological evaluation of Stafne bone defect are analyzed together with a review of the literature.
CASE REPORT
A 65- year old male reported to our department with a chief complaint of a painless swelling on the left side of the face that had been enlarging progressively over the past six years. (Figure 1a) There was no history of local trauma, radiation, surgery, or infection on that side of his face and neck. The patient was positive for diabetes and hypertension. However the family history was unremarkable.

Physical examination revealed a firm, well circumscribed, lobulated superficial mass of 15cm in diameter in the left parotid region. The mass was movable and was independent of the jaw function. The left ear lobule appeared elevated (Figure 1b). There was no sign of paresthesia, facial nerve weakness or cervical lymphadenopathy. The overlying skin appeared normal in colour and texture. The patient was edentulous and no significant intra-oral findings were present ( Figure 2).

On the basis of clinical findings a provisional diagnosis of benign salivary gland tumour was made. Differential diagnoses included pleomorphic adenoma, myoepithelioma or basal cell adenoma.
The pantomograph revealed cortical erosion near the left angle and ramus region, but also a well defined, corticated, oval radiolucency of size 1.5cm χ 1.0cm near the right angle region below the inferior alveolar canal, suggestive of a Stafne bone defect (Figure 3). No significant related intraoral findings were evident and the patient had no specific complaints.

Ultrasound examination of the swelling demonstrated a well circumscribed lesion measuring 15cm χ 10.5cm χ 7.5cm on the left side of face, showing mild vascularity.
The parotid gland was not visualized separately from the lesion. Left submandibular gland could be visualized separately, with the lesion abutting its lateral surface (Figure 4). The patient underwent fine needle aspiration (FNA) cytology. The smears showed tight clusters of small groups of cells with a micro-acinar arrangement. Cells were bland with round nuclei and moderate amount of cytoplasm. A few stromal fragments were also seen. These features suggested a benign parotid gland tumour.

Magnetic resonance imaging (MRI) was performed with a 1.5 T magnet using a dedicated neck coil. MRI and CT images of the left parotid region reveal a rounded, well defined mass measuring 9.5cmx 11.7cm x11.5cm, which appeared to be encapsulated and was arising from the superficial lobe of left parotid gland with no involvement of facial nerve. A slight heterogenous enhancement was seen when contrast medium was used in one of the CT images, but, when used in one of the short T1 inversion images (STIR), the technique revealed a moderately heterogeneous enhancement, an effect often indicative of malignancy, although the features and extensions suggested benign neoplastic lesion of left parotid region. MRI and CT of the right angle region of the mandible showed a defect. It contained soft tissue that was continuous with the adjacent submandibular gland, and was identical in signal intensity to the gland on both sequences. A diagnosis of Stafne bone defect was made, and no further therapy was instituted (Figures 5 and 6).

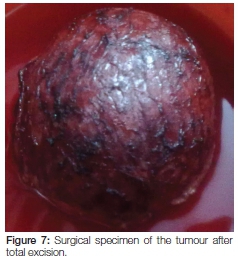
Chest radiograph of the patient was also normal. All investigations related to left parotid region suggested a benign etiology. The patient was committed to surgery and, using a standard parotid incision, a total parotidectomy was performed, preserving the facial nerve. The tumour was totally excised (Figure 7) with safety margins and the tissue sent for histopathological examination.
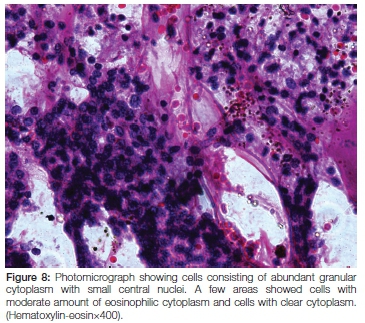
Microscopy showed tumour cells arranged in sheets and nested arrangements separated by fibrovascular stroma. The cells had abundant granular cytoplasm and small central nuclei, with a few areas demonstrating cells with a moderate amount of eosinophilic cytoplasm and others with clear cytoplasm. Extensive necrosis and haemorrhage was a feature. The capsule was thick and collagen-ous. No evidence of vascular invasion was seen within the tumour, nor was there any indication of abnormal mitoses. The final histopathological diagnosis was acinic cell carcinoma of the left parotid gland (Figure 8).
Postoperative recovery was uneventful (Figure 9). The patient received 20 cycles of radiotherapy and remains in good health three years later without any evidence of residual or recurrent disease.

DISCUSSION
ACC is a rare malignancy. It affects women more frequently (58.8%) than men (41.2%).The median age at diagnosis is younger than for most other salivary gland cancers (52 years).10,11 The median tumour size is two centimeters.10,11 Metastasis (regional or distant), high grade, and large tumour size are all more common among patients older than 30.10,11 Despite the tumour in this case being large (15cm), no regional or distant metastasis was evident. Possible causes of ACC include previous radiation exposure, familial predisposition and hormonal disturbances.1 No such risk factors were identified. Facial nerve palsy (3.0%) and pain (7.5%) are reported infrequently.4 The patient in this instance was asymptomatic and no sign of facial palsy was present in spite of the large size of the lesion. Immunohistochemical staining of ACC is not specific and has an insignificant role in diagnosis.12 Acinic cell carcinoma are graded according to their malignant potential.13 Low-grade malignancies (Grade I) are completely encapsulated with no capsule invasion. Signs of capsular invasion are present in moderate (Grade II) malignancies. Infiltration of the surrounding tissues and papillary cystic zones are present in high grade (Grade III) tumours. Adjacent tissue invasion, regional lymphatic dissemination and distant metastasis (bone and lungs) are possible.13 Guimaraes et al classified ACC based on cellular pleomorphism and presence of necrosis and / or mitosis. Grade I tumours exhibit mild to moderate cellular pleomorphism without necrosis and/or mitosis. Grade II tumours demonstrate only focal necrosis and/or 1 to 9 mitoses per 20 highpower fields, independent of the degree of cellular pleomorphism present. Grade III exhibit massive areas of necrosis and/or more than 10 mitoses per 20 high-power fields regardless of the degree of cellular pleomorphism.14 Our case was rated as Grade I based on malignant potential and Grade III on the basis of necrosis and cellular pleomorphism. Histologically, acinic cell carcinoma are of four types; (a) solid (b) papillary cystic (c) follicular (d) microcystic. A combination of these patterns usually occurs with predominance of one group.15 The present case represented the solid lobular type of ACC.
Preoperative diagnosis of ACC of the parotid gland is difficult. FNA has a specificity of 86% to 96% and a sensitivity of 82% to 91% but it can also be deceptive.16 A CT scan usually exhibits slight contrast enhancement and may be necessary for evaluation of tumour size, the relationship to facial nerve and other structures, extension and distant metastasis. MRI scan usually demonstrates a nonspecific signal intensity pattern which can be similar to the signal produced by some benign salivary neoplasms.17, 18
Treatment involves complete surgical removal of the tumour, by superficial or total parotidectomy. Recurrent, undifferentiated cases of ACC, positive margins, and advanced tumours with cervical lymph node spread may require postoperative radiotherapy.10,11,19,20 North et al recommended postoperative radiotherapy for all cases of salivary gland cancer except for those tumours staged as T1N0 or T2N0 with low-grade histology, which are successfully excised with negative margins.21 The treatment proposed in the present case was surgical excision and postoperative radiotherapy. ACC are distinctive neoplasms of typically unpredictable behaviour.10,11 Generally the five-year disease-specific survival is estimated to be around 91%, and at 10 years, 88%.22 The five-year survival rate for high-grade tumours, however, is only 33%.3
Stafne bone defect, also known as static bone cyst, lingual mandibular bone defect, Stafne bone cavity, idiopathic bone cavity, and lingual mandibular bone depression is the only described bone lesion that is highly localized, nonprogressive and yet non-healing.9,23 They may account for an extraosseous course of the mandibular neurovascular bundle.24 The size of the defect may range from 5mm to 90mm.25 Radiographically, the cortical outline of the bone defect is thicker and denser than that of odontogenic cysts.26 Intraoral dental films or pantomograph, although sufficient for diagnosis, may not be definitive in atypical lesions (eg, incompletely corticated, lobulated, multiple, or in an uncharacteristic location).7 Confirmatory testing is warranted in these situations, as the differential diagnosis for mandibular radiolucencies includes periapical cyst, traumatic bone cyst, odontogenic keratocyst, dentigerous cyst, fibrous dysplasia, ameloblastoma, vascular malformation, nonossifying fibroma, focal osteoporotic bone marrow defect etc.7 Stafne bone defects have revealed salivary tissue on most CT evaluations.27,28
However radiation exposure and possible contrast reactions are drawbacks of CT.7The diagnosis of a Stafne bone cavity without intravenous contrast material is feasible on the basis of the inherent soft-tissue contrast on both CT and MRI studies.7 Some authors advocate MR imaging as the primary diagnostic technique.29 MRI evaluation on both proton density and T1-weighted sequences showed the mandibular defect in this case to contain soft tissue continuous and isointense with the submandibular gland, thus confirming salivary tissue within the defect. Surgery is not required for the treatment of Stafne bone defect.29
Biopsy or surgical exploration should be conducted in atypical cases or other suspected lesions.29 The bone defect of our patient was asymptomatic and the possibility of any other bone lesions, such as cysts and tumours was excluded because there were no apparent signs of inflammatory or malignancy changes. Therefore, surgery was not considered. The patient was followed for three years. No remarkable changes of the defect was seen during the follow-up period; thus justifying the accuracy of the initial diagnosis of Stafne bone defect.
This association of Stafne bone defect and acinic cell carcinoma has been reported for the first time in literature. The pathogenesis of these entities may have a role to play in their occurrence. Long term follow up is mandatory in such cases.
Declaration: No conflict of interest declared
ACRONYMS
ACC: acinic cell carcinoma
CT: Computed Tomograph
FNA: fine needle aspiration
IV: Intravenous
MRI: Magnetic Resonance Imaging
STIR: short T1 inversion image
References
1. Al-Zaher N, Obeid A, Al-Salam S, Al-Kayyali BS. Acinic cell carcinoma of the salivary glands: a literature review. Hematol Oncol Stem Cel Ther 2009; 2(1): 259-64. [ Links ]
2. Cha W, Kim MS, Ahn JC, Cho SW, Sunwoo W, Song CM et al. Clinical analysis of acinic cell carcinoma in parotid gland. Clinical and Experimental Otolaryngology 2011; 4(4):188-92. [ Links ]
3. Federspil PA, Constantinidis J, Karapantzos I, Pahl S, Markmann HU, Iro H. Acinic cell carcinomas of the parotid gland. A retrospective analysis. HNO. 2001; 49(10):825-30. [ Links ]
4. Spiro RH, Huvos AG, Strong EW. Acinic cell carcinoma of salivary origin: Aclinicopathologic study of 67 cases. Cancer. 1978; 41:924-35. [ Links ]
5. Foote FW Jr, Frazell EL. Tumours of the major salivary glands. Cancer. 1953; 6(6):1065-133. [ Links ]
6. Stafne EC. Bone cavities situated near the angle of mandible. J Am Dent Assoc 1942; 29:1969-72. [ Links ]
7. Branstetter BF, Weissman JL, Kaplan BS. Imaging of a Stafne bone cavity: What MR adds and why a new name is needed. AJNR Am J Neuroradiol 1999; 20:587-9. [ Links ]
8. Nikzad S, Azari A, Dhezri FH. Diagnosis of a lingual mandibular bone defect (Stafne's bone Defect) by CT scan. Iran J Radiol 2010; 7(1):27-30. [ Links ]
9. Krafft T, Eggert J,Karl M. A Stafne bone defect in the anterior mandible- A diagnostic dilemma. Quintessence Int 2010; 41:391-3. [ Links ]
10. Hoffman HT, Karnell LH, Robinson RA, Pinkston JA, Menck HR. National Cancer Data Base Report on cancer of the head and neck: acinic cellCarcinoma. Head and Neck. 1999; 21(4):297-309. [ Links ]
11. Stewart AK, Bland KI, McGinnis LS, Morrow M, Eyre HJ. Clinical highlights from the National Cancer Data Base. CA Cancer J Clin. 2000; (50):171-83. [ Links ]
12. Gomez DR, Katabi N, Zhung J, Wolden SL, Zelefsky MJ, Kraus DH, et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009 15; 115(10):2128-37. [ Links ]
13. Byers RM, Piorkowski R, Luna MA. Malignant parotid tumours in patients under 20 years of age. Arch Otolaryngol 1984; 110: 232-5. [ Links ]
14. Guimaraes DS, Amaral AP, Prado LF, Naseimento AG; Acinic cell carcinoma of salivary glands; 16 cases with clinicopathologic correlation, J Oral Pathol Med 1989; 18: 396-9. [ Links ]
15. Rajendran R, Sivapathasundharam B. Tumours of Salivary Glands, Shafer's Textbook of Oral Pathology, 6th Edition, Elsevier, pages 219-31. [ Links ]
16. Michail P, Karavokyros I, Pikoulis E, Arvelakis A, Charminis G, Michail O, et al. Acinic cell carcinoma of the parotid gland in children : A case report and literature review. West Indian Med J 2008; 57 (1): 70-2. [ Links ]
17. Som PM, Bergeron RT. Salivary glands. Head and neck imaging. Mosby Year Book, St.Louis; 1991: 277-348. [ Links ]
18. Sakai O, Nakashima N., Takata Y, Furuse M. Acinic cell carcinoma of the parotid gland: CT and MR I. Neuroradiology 1996; (38): 675-9. [ Links ]
19. Kane WJ, McCaffrey TV, Olsen KD, Lewis JE. Primary parotid malignancies. A clinical and pathologic review. Arch Otolaryngol Head Neck Surg 1991; 117(3):307-15. [ Links ]
20. Colmenero C, Patron M, Sierra I. Acinic cell carcinoma of the salivary glands. A review of 20 new cases. J Craniomaxillofac Surg 1991; (19): 260-6. [ Links ]
21. Acinic Cell Carcinoma Overview, Acinic Cell Carcinoma Information Centre. [ Links ] [homepage on the Internet] [updated 2005 July 16; cited 2008 Sep 23]. Available from: http://www.acin-iccell.org/overvview.html
22. Wahlberg P, Anderson H, Biorklund A, Moller T, Perfekt R. Carcinoma of the parotid and submandibular glands-a study of survival in 2465 patients. Oral Oncol 2002; 38(7):706-13. [ Links ]
23. Grellner TJ, Frost DE, Brannon RB. Lingual mandibular bone defect: report of three cases. Journal of Oral and Maxillofacial Surgery 1990; 48(3): 288-96. [ Links ]
24. Reuter I. An unusual case of Stafne bone cavity with extraos-seous course of the mandibular neurovascular bundle. Dentomaxillofac Radiol 1998; 27:189-91. [ Links ]
25. Apruzzese D, Longoni S. Stafne cyst in an anterior location. J Oral Maxillofac Surg 1999; 57:333-338. [ Links ]
26. Campos PS, Oliveira JAC, Dantas JA, et al. Stafne's defect with buccal cortical expansion: a case report. International Journal of Dentistry, 2010, Article ID 515931, 3 pages, 2010. [ Links ]
27. Ariji E, Fugiwara N, Tabata O, et al. Stafne's bone cavity: classification based on outline and content determined by computer tomography. Oral Surg Oral Med Oral Pathol 1993;76: 375-80. [ Links ]
28. Slasky BS, Bar-Ziv J. Lingual mandibular bone defects: CT in the buccolingual plane. J Comput Assist Tomogr 1996;20:439-43. [ Links ]
29. Munevveroglu AP, Aydin KC. Stafne bone defect: report of two cases. Case Reports in Dentistry Volume 2012, Article ID 654839, 5 pages. [ Links ]
 Correspondence:
Correspondence:
Η Sabir
Tel: +91 964 457 7753
E-mail: husain_sabir2000@yahoo.com













