Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.70 no.3 Johannesburg abr. 2015
CASE BOOK
Oral medicine case book 68: Oral ulceration caused by rifampicin-resistant tuberculosis
MT PeckI; J HilleII; A SnymanIII; WP DreyerIV
IBChD, MScDent, MRD RCSEd(Perio), MChD(OMP). Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape
IIDDS, MDent, FCPath(SA). Division of Oral Pathology, Faculty of Dentistry, University of the Western Cape & National Health LaboratoryService, Tygerberg
IIIMBChB(Stell). Private Practice, Cape Town
IVBDS, HDipDent, PhD, FCD(SA)OMP Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape; Professor Emeritus, Stellenbosch University
CASE REPORT
A 53-year old female was referred by her local general medical practitioner to an oral medicine specialist for the management of a persistent ulcer on the left side of her tongue. The lesion had been present for at least three months and was not responding to treatment by topical antiseptic agents. The earlier removal of a molar in close proximity to the lesion, in an attempt to exclude the possibility of traumatic ulceration, had also yielded no beneficial effects. Upon examination, the patient appeared clinically healthy but presented with a history of emphysema due to chronic cigarette smoking. The emphysema was currently being managed by oral inhalation steroids. Even though smoking cessation had previously been advised, she failed to comply and was currently still smoking more than 10 cigarettes per day.
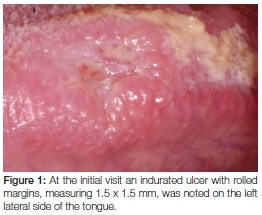
Her intraoral examination revealed signs of a healing extraction socket in the lower left molar area and brown staining of the dorsal surface of the tongue. A 1.5 x 1.5cm ulcer with rolled margins and signs of induration was noted on the left lateral surface of the tongue opposite the left lower first molar (Figure 1). Interestingly, no detectable lymphadenopathy was noted. Based on the history and clinical findings, a differential diagnosis of neoplasia, tuberculosis or chronic trauma associated ulceration was made.
An incisional biopsy that included the margins of the lesion was carried out under local anaesthesia to confirm the clinical diagnosis. At the same time, the patient was advised to reduce smoking to decrease her cancer risk as well as to the improve healing of the biopsy site. The histopathologic examination of the biopsy revealed ulcerated mucosa with a florid chronic granulomatous infiltrate in the submucosa.
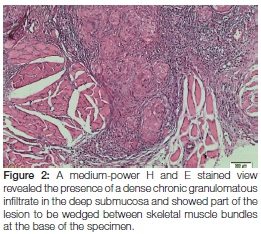
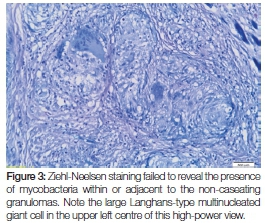
Whilst there were no signs of caseating necrosis, the presence of a few multinucleated (typical tuberculosis-associated) Langhans-type giant cells was, however, noted and the granulomatous inflammation showed infiltration by lymphocytes and numerous eosinophils. Of pertinent interest was the fact that the granulomas extended deeply into the submucosa and were wedged between the skeletal muscle fibres at the base of the specimen (Figure 2). The presence of eosinophils could be attributed to chronic skeletal muscle damage. No signs of epithelial dysplasia or neoplasia were noted. Ziehl-Neelsen staining was negative for mycobacterial species (Figure 3) and Periodic acid-Schiff staining did not expose signs of fungal infection. Immunoperoxidase studies for cytokeratins were negative, thus ruling out a malignant squamous infiltrate which could have been masked by the dense granulomatous inflammation. The histological diagnosis of chronic granulomatous inflammation, that could not be further specified, was made.
Due to the inconclusive nature of the histopathological findings, additional investigations were requested to exclude systemic disease and specifically tuberculosis. This revealed the following significant findings: An ESR of 58; normal full blood and differential white blood counts; negative test for HIV; negative results for syphilis; plain chest radiographs indicating a miliary pattern of lung calcifications (also noted on radiographs taken two years earlier); and high resolution computer tomography (CT) showing lung calcifications that were considered non-specific by the radiologists but were deemed to indicate a possible infectious granulomatous disease. The differential diagnosis was consequently altered to include tuberculosis, varicella pneumonia and sarcoidosis.
Based on these investigations, the original paraffin-embedded tongue tissue sample was subjected to further analysis using Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) which is a cartridge-based, automated diagnostic test using a polymerase chain reaction (PCR) based assay. The results confirmed the presence of tuberculosis DNA from a strain that was resistant to rifampicin and consequently a final diagnosis of an oral ulcer secondary to rifampicin-resistant tuberculosis (RMR-TB) was made.
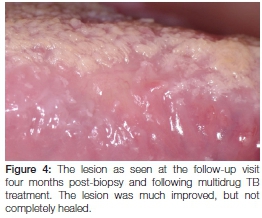
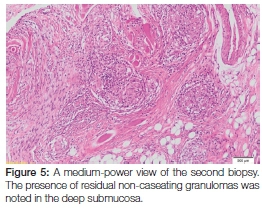
As a result of this diagnosis, the patient was promptly referred back to her GP who, following protocol, referred her for treatment at her local community health centre. Here she received four months' worth of intensive multidrug-resistant tuberculosis therapy that included a cocktail of medication including kanamycin (injectable), moxifloxacin, pyrazinamide, ethambutol and ethionamide. At her four month follow-up appointment, she reported feeling better and the original oral ulcer showed a marked decrease in size (Figure 4). Upon request from her treating physician, a second biopsy of the site was taken at this time to exclude active disease. The histopathology revealed the presence of residual non-caseating granulomas containing Langhans giant cells and lymphocytes within the deeper submucosa (Figure 5). The Ziehl-Neelsen stain was again negative for mycobacterial species, however, the possible diagnosis of (residual) tuberculosis was suggested. The patient was subsequently requested to return routinely in order to monitor the oral lesion as well as to detect the possible development of any new lesions. Because of the prolonged treatment time associated with this condition, she was still undergoing anti-tuberculosis therapy at the time of publication.
DISCUSSION
Oral lesions caused by the bacillus Mycobacterium tuberculosis may arise as primary lesions or, more commonly, as lesions secondary to pulmonary tuberculosis. The escalation of tuberculosis (TB) in many areas of the world, and especially in the Western Cape of South Africa, could produce an increase in the prevalence of oral lesions.1 Multidrug-resistant TB is also becoming more prevalent and oral lesions caused by multidrug resistant strains of the bacillus should thus, at least theoretically, also be on the rise. However, the present case is one of the first to be reported.
Rifampicin-resistant tuberculosis (RMR-TB) is a rapidly emerging form of TB, characterised by resistance to the anti-TB drug, rifampicin (RIF). RIF is an important first-line drug in the treatment of TB and acts by arresting the DNA-directed RNA synthesis of M. tuberculosis.2Once regarded as rare, RMR-TB appears to be on the rise, especially in areas like the Western Cape, South Africa.3,4 This is significant, since multidrug-resistant TB (MDR-TB) is often preceded by the acquisition of resistance to either isoniazid (INH) or RIF.4-6 Individuals co-infected with TB and HIV (human immunodeficiency virus), HIV infected individuals on antiretroviral therapy and those with a previous history of TB treatment appear to be at increased risk for acquiring RMR-TB infection.3-5 Alcohol abuse is probably another risk factor associated with the disease.4 Patients in countries with a high usage of rifampicin-type drugs for the management of bacterial infections, also appear to be at increased risk.7 As with other forms of tuberculosis, RMR-TB is thought to be transmitted via close contact and at least three different studies, from disparate regions, confirm this tendency.3-5 In the case presented above, the only identifiable TB contact was a close relative who had been treated successfully for TB 15 years previously.
The mechanism of RIF resistance has recently been studied. Pang et al (2013) showed that specific genetic mutations located in the 81-bp region (RIF resistance-determining region) as well as the increased transcription of several RIF-related efflux pumps, may be responsible for a large percentage of the RIF-resistant TB strains currently seen.2 Efflux pumps are primitive transport proteins located in the cytoplasmic membrane of most cells, including bacteria. These pumps are involved in the extrusion of intracellular substances into the external environment and are often associated with the efflux of multiple substrates, including antibiotics.8 Although their physiological function is not completely understood, the over-expression of efflux pumps is thought to be a major reason for antibiotic resistance. This resistance may not be antibiotic specific but may also incur resistance to other substances, such as dyes, detergents, and disinfectants (including triclosan). Although one might presume that over-expression of these pumps may occur as a result of exposure to antibiotic stresses, this may not be the case. It is presupposed that over-expression may occur due to mutations of local suppressor genes resulting in the development of bacterial species with a high efficacy of resistance to a number of substances. This may also explain the high intrinsic antibiotic resistance of certain bacterial species.8 The clinical significance of these antibiotic resistant species therefore warrants the use of a broad spectrum of drugs to effect a significant clinical change.
DIAGNOSIS AND TREATMENT
The diagnosis of drug-resistant TB is based on clinical and laboratory examination. Once TB is suspected, based on clinical, histological and/or microbiological grounds, two types of tests are used to evaluate drug susceptibility.9 These include WHO-endorsed solid or liquid culture-based tests, such as solid agar or Bactec MGIT 960 (Becton-Dickson, Sparks, MD, USA), and molecular based tests that include nucleic acid amplification (NAAT).9 Culture based tests assess the inhibition of M. tuberculosis growth in the presence of antibiotics10 and even though they are regarded as the diagnostic gold standard, culture based tests suffer from a number of disadvantages. These include the time required (minimum 21 days), the specialised equipment needed and the lack of infrastructure in most resource-poor settings.8-9 In the above case, culture based tests were not possible due to the limited size of the tissue biopsy.
Unlike culture-based tests, NAATs allow much faster processing times, with results often available within two hours.9 The most commonly used NAAT is Xpert® MTB/ RIF (Cepheid, Sunnyvale, CA, USA). This is a polymerase chain reaction test that simultaneously diagnoses TB and RIF resistance, and is now endorsed by the WHO as a first investigation for patients suspected of having drug resistant tuberculosis. Although rapid, the disadvantage of Xpert® MTB/RIF, is that is does not test for INH resistance. This may result in patients suffering from RIF mono-resistant-TB being overtreated.6 Another major disadvantage of Xpert® MTB/RIF is that it is associated with inconsistent results, especially for extrapulmonary cases.9, 11 A culture based test or another type of NAAT test would then be indicated to confirm the diagnosis.
TREATMENT
The management of patients suffering from RMR-TB is complex and is not fully standardized. International guidelines offer different opinions with some advocating management similar to that of MDR-TB. This consists of a backbone of later generation fluoroquinilone as well as an injectable aminoglycoside, together with any first line drug to which the bacterium is susceptible. In addition to this, an oral bacteriostatic second-line TB drug should also be used. Treatment is initiated by an intensive phase of injectable medication that might last up to 18 months with the total duration of treatment usually extending up to 24 months.3-5 Because of the prolonged treatment regimen, patients have to be monitored frequently for compliance, adverse drug reactions and drug interactions. The treatment outcome for RMR-TB varies according to geographical location, regimen choice and duration of treatment. Curative rates of up to 67% have been recorded but may be influenced by the factors mentioned above.5
CONCLUSION
The case presented above has clinical significance for the oral health care practitioner. Oral lesions may be the first sign of systemic disease and, as in this case, a non-healing ulcer was the only presenting sign of RMR-TB. Thus, although oral ulceration is common, and TB associated oral ulceration is a well-documented phenomenon, the rise of resistant TB strains warrants careful consideration when managing these patients. The varied curative rate as well as the potential for disease spread must be taken into account. Practitioners must be aware that in order to effectively manage these complex conditions, interaction with the appropriate specialists, as well as with other healthcare workers, may be required. An acute awareness of systemic disease is a prerequisite for diagnosing oral disease, especially in cases with potentially fatal disorders.
Declaration: No conflict of interest declared
ACRONYMS
DNA: Deoxyribonucleic acid
HIV: Human immunodeficiency virus
INH: Isoniazid
MDR-TB: Multidrug-resistant tuberculosis
NAAT: Nucleic acid amplification test
PCR: Polymerase chain reaction
RIF: Rimfampicin
RMR-TB: Rimfampicin-resistant tuberculosis
RNA: Ribonucleic acid
TB: Tuberculosis
References
1. Kakisi OK, Kechagia AS, Kakisis IK, et al. Tuberculosis of the oral cavity: a systematic review. Eur J Oral Sci 2010; 118: 103-9. [ Links ]
2. Pang Y, Lu J, Wang Y, Yuanyuan S, Wang S, Zhao Y. Study of the rifampin mono-resistance mechanism in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013; 57: 893 [ Links ]
3. Ridzon R1, Whitney CG, McKenna MT, et al. Risk factors for rifampin mono-resistant tuberculosis. Am J Respir Crit Care Med 1998; 157: 1881-4. [ Links ]
4. Mukinda FK1, Theron D, van der Spuy GD, et al. Rise in rifampicin-monoresistant tuberculosis in Western Cape, South Africa. Int J Tuberc Lung Dis 2012; 16: 196-202. [ Links ]
5. Meyssonnier V, Bui TV, Veziris N, Jarlier V, Robert J. Rifampicin mono-resistant tuberculosis in France: a 2005-2010 retrospective cohort analysis. BMC Infect Dis 2014; 10: 14-8. [ Links ]
6. Kurbatova EV, Cavanaugh JS, Shah NS, et al. Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis 2012; 16: 355-7. [ Links ]
7. Jarallah JS1, Elias AK, al Hajjaj MS, Bukhari MS, et al. High rate of rifampicin resistance of Mycobacterium tuberculosis in the Taif region of Saudi Arabia. Tuber Lung Dis 1992; 73: 113-5. [ Links ]
8. Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 2003, 51: 9-11. [ Links ]
9. Calligaro GL, Moodley L, Symons G, Dheda K. The medical and surgical treatment of drug-resistant tuberculosis. J Thorac Dis 2014; 6: 186-95. [ Links ]
10. Migliori GB, Matteelli A, Cirillo D, Pai M. Diagnosis of multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: Current standards and challenges. Can J Infect Dis Med Microbiol 2008; 19: 169-72. [ Links ]
11. Ozkutuk N, Surucüoglu S. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary tuberculosis in an intermediate-prevalence setting. Mikrobiyol Bul 2014; 48: 223-32. [ Links ]
 Correspondence:
Correspondence:
WP Dreyer
PO Box 1285, Sedgefield, 6573
E-mail wpdreyer@telkomsa.net













