Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.70 n.3 Johannesburg Apr. 2015
RESEARCH
Microbial contaminants on dental bib chains with attached clips
J MolepoI; M MolaudziII; TRMD RalephenyaIII
IPhD (Medical Microbiology). Head of Department, Oral Biological Sciences, School of Oral Health Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMSc (Medical Microbiology). Senior Technician, Department of Oral Biological Sciences, School of Oral Health Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIOral Hygienist, MA (Developmental Studies). Head: Oral Hygiene Division, Department of Community Dentistry, School of Oral Health Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
INTRODUCTION: Reusable dental bib chains are considered to carry a low risk for transmission of infection, and are therefore simply cleaned and disinfected between patients. There is a paucity of information in South Africa on the potential risk of cross infection.
AIM: To investigate the extent of microbial contaminants present on such bib chains, after their use on patients having dental treatment and again after they had been disinfected.
METHODS: Tests were conducted on forty-four reusable metal bib chains with attached clips which had been used in patient treatment at the Wits Oral Health Centre. The bacterial contaminants remaining on used, and then later disinfected, bib chains were assessed. Microorganisms were identified using conventional microbiological methods. Data were analyzed using STATA 11 software.
RESULTS: A high number (84.1%) of the used bib chains harbored microbial contaminants after dental treatment. Thirty-four percent of the bib chains still carried microbial contaminants after disinfection. Enteric, environmental and skin bacteria were observed.
CONCLUSION: The used bib chains in this study carried potentially infectious microorganisms. The disinfection.
Key words: Dental treatment, Microbial contaminants, Bib chains, Disinfection
INTRODUCTION
During a dental procedure, the chains holding the protective bib come into contact with aerosols, hair and skin, and with saliva, blood and other oral substances that spray out of the mouth.1 Re-useable dental bib chains are considered to carry a low risk for transmission of infection, and standard procedures, followed by Dental Practitioners, are therefore that they need only be cleaned and disinfected between patients.2
Very few studies have been conducted on dental bib chains and their disinfection. Molinari3 reported microbial contamination of metal and plastic bib holders post-treatment and post-disinfection, and warned that repeated use of bib chains amongst patients may increase the number of pathogens on the bib chain. In a recent study, aerobic bacteria including Pseudomonas and Staphylococcus epidermidis, and anaerobic bacteria including Propionibacterium acne, Eikenella corrodens and Prevotella were identified on the chains after their use in treatment and were demonstrated again after disinfection of the bib clips.4
Alt-Holland et al.5reported that between 20% to 30% of the surfaces of bib clips carried microorganisms, even after disinfection. A study conducted at a university in the southeastern United States revealed Pseudomonas, Sta-phylococcus aureus and Escherichia coli present on the chains after dental treatment and after disinfection.6 Similarly, researchers in Germany found that 70% of bib holders remained contaminated after disinfection.7 It is suggested that the design of the bib chain has a role to play in the harboring of microorganisms as any cracks, crevices and indentations enhance the chances of an increase in the bacterial count on the bib chains.1
Whilst it may be likely that the South African experience may be similar, there is a paucity of local information which describes microbial contaminants on bib chains and the effect of disinfection of these bib chains. This study undertook such an investigation at an Oral Health Centre in South Africa.
MATERIALS AND METHODS
Study population and methodology
Tests were carried out on 44 reusable metal bib chains (Prodent, NJ, USA) used on patients presenting at the Wits Oral Health Centre. The study was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg (W-CJ-131104-1).
Sampling was done in the patient examination room and a total of 88 samples were collected (two from each reusable metal bib chain). The first (post-treatment) was collected immediately after oral examination and treatment of the patient had been completed. The chain was removed at once and was placed into 20ml of sterile tryptone soya broth (TSB; Sigma Aldrich, Steinheim, Germany). The mixture was then vortexed for 30 seconds to remove and collect microbial contaminants from the bib chain, which was then removed aseptically from the TSB and was disinfected by spraying with Tristel®-Chlorine Dioxide 99.99% (Wright-Millners, South Africa). The chain was then allowed to dry for one minute. Now the second sample (post-disinfection) was collected, following the same procedure as for the first.
Identification of Microorganisms
In the laboratory, each TSB suspension was centrifuged at 3000rpm for 5 minutes. The pellets were suspended in 2ml of TSB. One hundred microliters of each suspension was cultivated onto 5% horse blood agar (BA) using the spread test method,8 and incubated aerobically at 370C for 48 hours. Sterile unused TSB served as a negative control. All the colonies which developed were counted, sub-cultured and identified, based on bacterial characteristics, morphology and Gram staining. Catalase and coagulase tests were used to identify gram positive cocci. Staphylococcus aureus identification was confirmed using DNAse test and culture on MSA agar. Gram-negative bacilli were subcultured onto MacConkey plates and tested with API20E according to the manufacturer's directions (bioMérieux, Ltd, Basingstoke, UK).
DATA ANALYSIS
Data were analyzed using the Stata statistical package (STATA 11 for Windows; Stata Corp., College Station, TX, USA). All statistical significance was calculated at the 5% significance level.
RESULTS
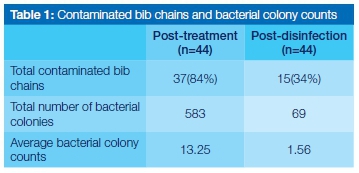
A large number of bib chains were shown to be contaminated with microorganisms post-treatment (84%), which reduced to 34% post-disinfection.
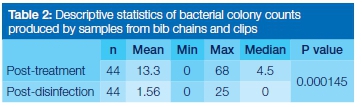
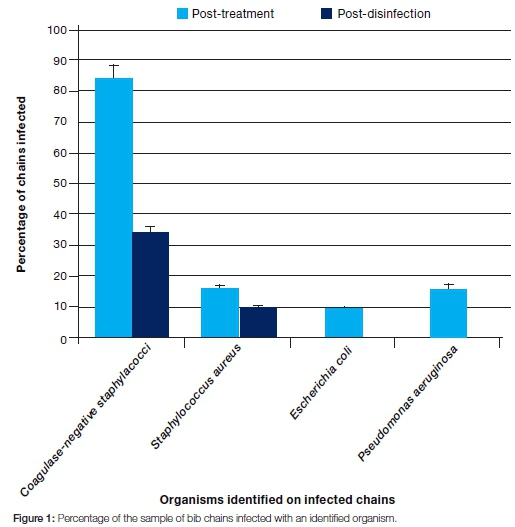
The total number of bacterial colonies observed post-treatment was considerably higher (583) than that observed post-disinfection (69) (Table 1). There was a statistically significant difference (p=0.000145) between colony counts done at the post-treatment and post-disinfection stages (Table 2). Coagulase-negative staphylococci were the most dominant organisms identified in the post treatment samples (on all the infected chains, ie 84% of the total chains), followed by Staphylococcus aureus (16% of the chains) and Pseudomonas aeruginosa (16% of the chains). Escherichia coli was the least commonly isolated organism (9% of the chains). After disinfection, coagulase-negative staphylococci were again the most dominant (34% of the infected chains), followed by Sta-phylococcus aureus (9% of the chains). Escherichia coli and Pseudomonas aeruginosa were not observed after disinfection (Figure 1).
DISCUSSION
As metal bib chains and their clips are always used together at this Health Centre, they were investigated as one unit, in contrast with other studies where they were tested individually.1,3-5,7
The finding that 84% of the bib chains and clips in this sample carried potentially infectious microorganisms after their use during dental treatment procedures agreed with the results of previous studies.3,5-7 Contamination of bib chains with skin bacteria (coagulase-negative sta-phylococci and Staphylococcus aureus), environmental bacteria (Pseudomonas aeruginosa) and enteric bacteria (Escherichia coli) could have been caused by prolonged contact with the patient's neck with subsequent acquisition of normal skin flora, by exposure to contaminated aerosols and spatters during treatment, as well as during the handling of bib chains with contaminated gloves.
Coagulase negative staphylococci, which are normal inhabitants of the skin,9 were the most frequently identified microorganisms at both the after treatment (84%) and the post-disinfection (34%) stages. Similar results have been reported in a previous study.4 The possibility of cross-contamination between patients is of concern, as infections caused by these organisms are increasingly reported, especially in immuno-compromised patients.1011
The continued presence of Staphylococcus aureus after disinfection is serious, as these bacteria have been implicated in respiratory infections in immuno-compromised patients,12 and may become resistant to many antibiotics, leading to life-threatening infections.13
Escherichia coli and Pseudomonas aeruginosa, found to be present after the delivery of treatment, can survive in water-lines,2 and therefore contamination of chains and clips is quite feasible. Infection by these organisms may also lead to respiratory disease, again especially in immuno-compro-mised patients.14E. coli is a gram-negative bacterium commonly found in the intestines of animals and humans, and is transmitted via the faecal-oral route, causing disease. The organism can survive outside the body, making it an ideal indicator organism for testing for faecal contamina-tion.15 The observation in the current study of the presence of this organism after treatment may indicate contamination of or insufficient treatment of the water reservoir supplying the dental units. Further studies are indicated. It is of some reassurance that this study showed that these organisms were successfully eliminated from bib chains after disinfection, in contrast to previous reports.4,6
Although bacterial contaminants were not observed on the majority of bib chains after disinfection, 34% still carried contaminants. Nevertheless a statistically significant difference was shown between the bacterial counts on bib chains following treatment and after disinfection (p=0.000145). These findings indicate that disinfection with Tristel®-Chlorine Dioxide 99.99% was partially effective, completely eliminating some bacteria whilst allowing others to survive. This is in agreement with previous studies.4,5
The potential cross-contamination risks of these organisms which can cause respiratory infections in immuno-compromised patients is a cause for concern, especially in South Africa. South Africa has one of the largest HIV-infected populations in the world, with an estimated 5.6 million people living with HIV and AIDS.16
To reduce the risk of infection, we recommend, firstly, that bib chains be cleaned thoroughly before disinfection and/or sterilization to remove organic or inorganic material which could interfere with the inactivation of microbes. Failure to thoroughly clean bib chains could compromise the disinfection or sterilization processes.17 Secondly, we advise the use of alternative chemical disinfectants such as glutaraldehyde, which is effective and will not affect the skin or the bib chains. Alternatively, and most efficient, bib chains may be sterilized between patients, which will kill all microorganisms including spores. Various sterilization methods such as moist heat (autoclave), dry heat (hot air oven) or UV light can be used to sterilize bib chains.
CONCLUSION
This study demonstrated that bib chains and clips are readily contaminated by bacterial attachment during the delivery of dental treatment, with a high proportion (84%) shown to be harbouring enteric, environmental and skin bacteria. As the routine disinfection procedure was only partially effective, alternative procedures are recommended to minimize the risk of cross infection.
CLINICAL SIGNIFICANCE
Dental Practitioners should be aware that despite all their laudable efforts at achieving successful disinfection, their bib chains and clips pose a real risk of introducing cross infection. The risk may be aggravated in immuno-compro-mised patients, especially in South Africa, which has one of the largest HIV-infected populations in the world. There is a need to use alternative methods such as sterilizing bib chains in an autoclave or using disposable bib chains.
Acknowledgements
The authors are grateful to MR Ramoncha for her technical assistance and TB Mokale for assisting with the collection of samples. We thank the Department of Oral Biological Sciences, School of Oral Health Sciences, at the University of the Witwatersrand, for funding this project.
Conflict of interest
The authors declare no conflict of interest.
ACRONYM
TSB: tryptone soya broth procedure was only partially effective. These findings reveal the risk of potential cross infection of these microorganisms from patient to patient or from patient to healthcare worker.
References
1. Kelsch N. Don't Clip that Crud on Me. Reg Dent Hyg Mag. 2011; 30: 32. [ Links ]
2. Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Centres for Disease Control and Prevention. Guidelines for infection control in dental health-care settings. MMWR Recomm Rep. 2003; 52(RR-17):1-61. [ Links ]
3. Molinari JA. Microbial contamination of patient napkin holders. Dent Adv Res Rpt. 2010; RR#29. [ Links ]
4. Alt-Holland A, Murphy CM, Powers A, Kublin CL, Jeong YN, DIMattla M, Pham L, Park QA, Flnkelsteln M, Hanley JB, Paster BJ, Kugel G. Comprehensive analysis of aerobic and anaerobic bacteria found on dental bib clips at a hygiene clinic. Compend Contin Educ Dent. 2013; 34 (Supp):1-10. [ Links ]
5. Alt-Holland A, Srinivasan S, Lucier R, Kublin CL, Fong JM, Goldfein J, Park A, Finkel- man M, Kawai T, Paster BJ, Kugel G. Do bib clips pose a cross-contamination risk at the dental clinic? Compend Contin Educ Dent. 2012; 33:1-7. [ Links ]
6. Study finds bib chain a potential source of bacteria. Dent Health Mag; 2010. Available at http://worldental.org/dental-news/study-finds-bib-chain-potential-source-of-bacteria/1184/ (accessed 28 September, 2014). [ Links ]
7. Study: Bacteria found on 70% of dental bib holders [news release]. [ Links ] San Francisco, CA, 2012. Available at http://www.drbicuspid.com/index.aspx?sec=sup&sub=hyg&pag=dis&ItemID=310087 (accessed 28 September, 2014).
8. Koch AC. Growth measurement. In: Gerhardt P, Murray RGE, Wood NA, Krieg NR, editors. Methods for General and Molecular Bacteriology. Washington D.C: ASM Press; 1994. p.254-7. [ Links ]
9. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005; 43:5721-32. [ Links ]
10. Moller DV, Bruun NE. Substantial myocardial abscess in an immunocompromised patient: Fatal outcome after coagulase-negative staphylococcal native valve infection. J Am Soc of Echoc. 2007; 20:333.e7-e8. [ Links ]
11. Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009; 134:45-54. [ Links ]
12. Lobo LJ, Reed KD, Wunderink RG. Expanded clinical presentation of community- acquired MRSA pneumonia. Chest. 2010; 138:130-6. [ Links ]
13. Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005; 352:1445-53. [ Links ]
14. Denis C, Poirel L, Carricajo A, Grafford F, Fascia P, Verhoeven P, Gay P, Nordmann P, Pozzetto B, Berthelot P. Nosocomial transmission of NDM-1-producing Escherichia coli within a non-endemic area in France. Clin Microbiol Infect. 2012; 18: E128-E30. [ Links ]
15. Feng P, Weagant S, Grant, M (2002-09-01). "Enumeration of Escherichia coli and the Coliform Bacteria". Bacteriological Analytical Manual (8th ed.). FDA/Centre for Food Safety & Applied Nutrition. (accessed 15 September 2014). [ Links ]
16. UNAIDS. Regional fact sheet. 2011 UNAIDS W 1. World AIDS Day report. [ Links ]
17. Sterilization and Disinfection of Dental Instruments. http://www.ada.org/~/media/ADA/Member%20Center/FIles/cdc_sterilization.ashx (Accessed 07 January 2015). [ Links ]
 Correspondence:
Correspondence:
J Molepo
Department of Oral Biological Sciences, Faculty of Health Sciences, University of the Witwatersrand
Private Bag 3, Wits 2050. Johannesburg, South Africa.
Tel: 011 717 2229 Fax: 011 717 2121
Email: Julitha.Molepo@wits.ac.za













