Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.69 no.8 Johannesburg 2014
RESEARCH
Halitosis as a product of hepatic disease
M GuglielmiI; M BeushausenII; C FengIII; A BeechIV; D BaurV
IDDS. Oral and MaxilloFacial Surgery Attending Bronx Lebanon Hospital New York 1775 Grand Concourse, 6Th floor, Bronx, New York 10453
IIDMD. Case Western Reserve University Department of Oral and Maxillofacial Surgery. PGY 1
IIIPh.D. Associate Professor - Department of Biostatistics and Computational Biology University of Rochester Medical Center. Associate professor- Department of Anesthesiology (SMD). School of Medicine and Dentistry. Rochester, NY 14642
IVDMD. MetroHealth Hospitals Department of Oral and Maxillofacial Surgery. Non categorical intern
VD Baur: DDS. Case Western Reserve University Department of Oral and Maxillofacial Surgery. Department Chairman
ABSTRACT
OBJECTIVES: This study evaluated halitosis in patients suffering from hepatic disease.
MATERIAL AND METHODS: Twenty-five patients (12 males and 13 females) aged between 16 and 73 years who had undergone treatment for liver disease were included in this study. Three halimeter recordings were performed to measure methyl mercarptan and hydrogen sulphite. Mean values were calculated and compared with normal values (75-120 ppb). The level of significance was set at P < .05.
RESULTS: Thirteen of the 25 subjects (52%) had normal Volatile Sulphur Compound (VSC) values (75-120 ppb). Twelve subjects (48%) recorded values ranging from 132 to 1112 ppb. There was no correlation between hepatic pathology and halitosis. Fifty-two percent of all subjects had poor oral hygiene, strongly correlated with high VSC values (P<0,05) whereas the remaining 48% with good hygiene had normal levels of VSC.
CONCLUSIONS: Within the limitations of this study, high values of VSC were not associated with the presence of hepatic disease.
ACRONYMS
SAM: S-adenosyl methionine
VSC: Volatile Sulphur Compound
HCV: Hepatitis C virus
ppb: parts per billion
INTRODUCTION
Halitosis is a general word used to describe unpleasant breath, specifically in describing "bad" breath from the oral cavity.1 Scientific reports of halitosis first appeared in the 1930's2 but it was not until the mid-1960's that the definitive etiology, physiopathology and clinical therapy of halitosis was elucidated by Toenzetich,3,4,5 followed by further research in the 1990s by Rosenberg.6,7,8,9
The condition can be found in patients of any sex and age: females, males, children and the elderly,6,11Some studies have estimated the prevalence of bad breath in groups considered to be representative of the general population. Miyazaki et al12 concluded that 23% of the population presented with unpleasant breath. It is estimated that more than 50% of the adult population in North America suffer from halitosis.13
The Fourth World Conference on Halitosis in 1999 concluded that 85-90% of halitosis was caused by oral factors. In the remaining 10-15% of cases, halitosis has its origin in systemic diseases such as hepatic, pancreatic and renal insufficiencies, as well as trimethylaminuria, upper and lower respiratory tract infections, gastric content, and the consumption of some drugs.14
Halitosis is characterized by compounds such as Volatile Sulphur Compounds (VSC) which include hydrogen sulphide, methyl mercaptan, dimethyl sulphide and dimethyl disul-phide,3,5 volatile fatty acids with short aliphatic chains (i.e. butyric acid, valeric acid, isovaleric acid and propionic acid) and amines, (putrescin and cadaverin, indole and skatole).16 VSCs arise from bacterial metabolism of sulphur amino acids such as cysteine and methionine.17 Oral microorganisms that produce VSCs are mainly gram negative anaerobic periodontal pathogens such as Veillonella alcalescens, Porphyromonas gingivalis, Prevotella, intermedia, Prevotella, loeschii, Treponema denticola, Klebsiella pneumoniae, Fusobacterium nucle-atum and Eubacterium. These organisms produce hydrogen sulfide from L-cysteine and methylmercaptan from l-methio-nine.18 Intraoral conditions such as poor oral hygiene, periodontal pockets, biofilm of the tongue (especially the dorsal third), food impaction, unclean dentures, faulty restorations, oral carcinomas and throat infections favour the retention of anaerobic, mainly gram-negative, bacteria thus predisposing patients to the development of halitosis.19, 20, 21, 22
Numerous studies have focused on the relationship between halitosis and the bacteria in saliva or in dental plaque.23 Specific diseases such as acute necrotizing ulcerative gingivitis and pericoronitis have been shown to induce a distinctive bad breath associated with certain pathogenic gram-negative organisms. In addition, other oral conditions which have been associated with bad breath include aphthous ulcers, dental abscesses, candidiasis, oral cancers, and xerostomia.24
Halitosis appears to be a multi-factorial disease that requires a multidisciplinary approach in which dentistry plays an important role.10 Among the extra-oral predisposing factors for halitosis, fasting and morning breath are associated with temporary bad breath. This is the result of stagnation of epithelial cells and food debris combined with an overnight decrease in salivary flow,25 Certain hormonal changes that occur during ovulation, menstruation, pregnancy and menopause,26 as well as extra-oral diseases such as chronic sinusitis, tonsillitis, bronchitis, diabetes mellitus, hepatic failure, renal failure, and carcinoma of the lung have also been associated with oral malodour.24
There have been reports evaluating the presence of halitosis in patients with liver disease but these have not considered the periodontal status and oral hygiene.30, 31, 32
The objectives of this study were to assess the relationship between hepatic pathology and halitosis and also to evaluate the role of oral hygiene and condition.
MATERIAL AND METHODS
Twenty-five subjects, 12 males and 13 females, with a mean age of 59 years (range 16 to 73), undergoing medical treatment at the Department of Gastroenterology and Internal Medicine at the University La Sapienza, Rome, Italy, were included in this study, A dental history of the subjects was obtained, which included the oral hygiene status (assessed by the presence of plaque and calculus, gingival bleeding during tooth-brushing and gingival hyperplasia); self-assessment of breath or bad taste during the day; oral hygiene procedures (brushing; flossing; tongue scraping, use of mouth rinse); smoking and/or alcohol habits and diet (including the use of spices). Patients were classified as having poor oral hygiene if plaque was present in addition to at least two other clinical parameters i.e. calculus, gingival bleeding or gingival hyperplasia. Oral hygiene status was defined as good if neither plaque nor calculus were detected even in the presence of gingival bleeding and gingival hyperplasia.
Halitosis was assessed utilizing the Halimeter (Halimeter RH-17k, Interscan Corporation, Chatsworth, CA). The instrument is composed of a semiconductor sensor that catalyzes an electrochemical reaction, with production of an electric impulse directly proportional to the specific concentration of the Volatile Sulphur Compounds (VSC); a control panel, digital display, small Teflon tube for survey of the air sample, an engine pump to generate a vacuum for the aspiration of the oral air, an on/off switch and a calibration adjustment. The Halimeter measures the levels of VSC in a semi-quantitative manner, being especially sensitive to hydrogen sulphide. Dimethyl sulphide and methyl mercaptan are also detected, but the response of the instrument to these gases is about 50% less than it is to hydrogen sulphide. According to the manufacturer there is decreasing sensitivity in the following order: hydrogen sulphide > methyl mercaptan > dimethyl sulphide. The data was expressed in parts per billion (ppb) and the normal range is between 75-120 ppb.
Immediately prior to the examination the subject was instructed to keep his/her mouth closed for three minutes. A disposable 6.5 mm plastic straw was attached to the air inlet of the monitor. The subject was instructed to insert the straw into the oral cavity so that it extended approximately 4cm intraorally, ensuring that there was no contact with palate, teeth, tongue or cheeks. This prevented aspiration of substances such as saliva, blood and epithelial cells that could interfere with the quantification of VSC. A sample of air was aspirated through the plastic straw by the pump at 1500 mL/ min and then passed through the sensor which registered the levels of VSCs. The assessment lasted 10 seconds and the patient was instructed not to breathe through either the nose or mouth during the sampling. The peak value attained was recorded in ppb by direct readings from the analog scale of the monitor. The process was repeated three times, with a one minute interval during which the subject kept the mouth closed. The mean value of the three readings was calculated and correlated with the level of the oral hygiene status using PROC GENMOD in the SAS package (SAS Institute, Cary, North Carolina, version 9.1).
RESULTS
Twenty patients (80%) had hepatitis cirrhosis, 8%, chronic hepatitis C, 4% a hepatoblastoma, 4% had received a liver transplant, and 4% presented celiac disease. Of the patients with hepatic cirrhosis, 20% were crypto genetic, 30% were post-alcoholic and the remaining 50% had suffered from hepatitis C.
Table 1 illustrates the pathology, the associated halimeter values expressed in ppb and the status of oral hygiene.
Thirteen patients, i.e. 52%, had a mean VSC within normal values (75-120 ppb). Twelve subjects (48%) presented with halitosis and with escalated VSC values that ranged from 132 to 1112 ppb. However, no significant correlation was found between hepatic pathology and halitosis (Table 2). The strongest correlation was found between oral hygiene levels and halitosis (p< .001) (Table 3 & 4).


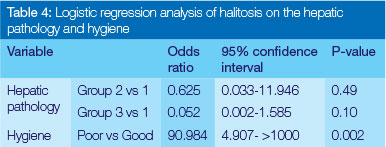
Forty eight percent of the patients, regardless of the type of hepatic pathology, had poor oral hygiene with severe plaque deposits, calculus and the presence of gingival bleeding. Poor oral hygiene levels were strongly correlated with high values of VSC (Table 5). The remaining 52% exhibited good hygiene and normal VSC values.

DISCUSSION
Halitosis has a complex multifactorial etiology. Researchers have suggested that 85-90% of patients with halitosis have an oral condition, whilst the remaining 10% have a non-oral origin.14 Systemic diseases i.e. diabetes mellitus, chronic renal failure, cirrhosis of the liver) are often associated with halitosis.27 Furthermore, medication i.e. chemotherapeutic drugs, psychiatric drugs and dimethylsulphide oxide may aid in the production of halitosis.28, 29
Methyl mercaptan has been isolated from the urine of a patient with massive hepatic necrosis and hepatic coma with fetor hepaticus, a condition seen in portal hypertension where portosystemic shunting allows mercaptans to pass directly into the lungs. This is a late sign in liver failure. Other possible causes are the presence of ammonia and ketones in the breath. Many authors have speculated that the odour may be a mixture of mercaptans and dimethyl disulphide.30 Together with cysteine, methionine is one of two sulphur-containing proteinogenic amino acids. Its derivative S-ade-nosyl methionine (SAM) serves as a methyl donor. Methionine plays a role in the biosynthesis of cysteine, carnitine, taurine and lecithin.
In the present study mercaptans were present in the breath of cirrhotic patients to approximately double the levels seen in normal subjects. This increase is probably due to two sources: the mercaptans formed by bacterial action in the intestine bypass the liver through shunts present in cirrhotic patients, and those formed in the process of cellular thiol metabolism, which the liver is unable to further metabolise.31
The liver also plays a major role in the utilization of fatty acids via the hepatic artery from fat deposits or other tissues via the portal vein from the intestinal tract. Hepatic disease causes damage or loss of liver tissue and may result in the accumulation of valeric acid, isovaleric acid and propionic acid as well as amines (putrescin and cadaverin, indole and skatole) all of which are components of halitosis.16
Sjogren's syndrome may also be considered one of the extra-hepatic manifestations of HCV35,36 Xerostomia causes plaque retention, and when combined with the stagnation of food debris, may generate halitosis.
This study further confirmed that poor oral hygiene was associated with halitosis.37,38
CONCLUSION
Within the limitation of this study, high values of VSC, and therefore halitosis, correlated with poor oral hygiene. No significant correlation was found between high VSC values and the presence of hepatic disease. Further research is needed to expand knowledge about halitosis using larger numbers of patients and controls.
References
1. Kleinberg I, Westbay, G. Oral Malodor. Crit Rev Oral Biol Med 1990;1: 247-59. [ Links ]
2. Prinz H. Offensive breath, its causes and its prevention. Dent Cosmos 1930;72:700-7. [ Links ]
3. Tonzetich J, Richter V, J. Evaluation of volatile odoriferous components of saliva. Arch Oral Biol 1964; 9:39-45. [ Links ]
4. Tonzetich J, Kestenbaum R. C. Odor production by human salivary fractions and plaque. Arch Oral Biol 1969;14: 815-27. [ Links ]
5. Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol 1971; 16: 587-97, [ Links ]
6. Rosenberg M, Kulkarmi GV, Bosy A, McCulloch CA. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991,70(11):1436-40. [ Links ]
7. Roesenberg M, Septon I, Eli I, Bar-Ness R, Gelemeter I, Brenner S, Gabby J. Halitosis measurement by an industrial sulphide monitor. J Periodontol 1991 ;62(8): 487-9. [ Links ]
8. Rosenberg M. Clinical assessment of bad breath: current concepts. J Am Dent Assoc. 1996;127(4):475-82. [ Links ]
9. Rosenberg M, Gabbay J. Halitosis a call for affirmative action. Refuat Hashinayim 1987;5(2):13-5. [ Links ]
10. Loesche WJ, Abrams J, Terpenning MS, Bretz WA, Dominguez BL, Grossman NS, Hildebrandt GH, Langmore SE, Lopatin DE. Dental findings in geriatric populations with diverse medical backgrounds Oral Surg Oral Med Oral Pathol Oral Radiol En-dod 1995;80(1):43-54. [ Links ]
11. Lin Mi, Flaitz CM, Moretti AJ, Seybold SV, Chen JW. Evaula-tion of Halitosis in Children and mothers. Pediatr Dent. 2003;25(6):553-8. [ Links ]
12. Miyazaki H, Sakao S, Katoh Y.Correlation between sulphur compounds and certain oral breath measurements in the general population. J Periodontol 1995;66:679-84. [ Links ]
13. Tessier JF, Kulkarni GV,, Bad breath: etiology, diagnosis and treatment. Oral Health 1991;81:19-22. [ Links ]
14. Preti G, Clark L, Cowart Bj, Feldman RS, Lowrry LD. Non oral etiologies of oral malodour and altered chemosensation. J Periodontol 1992; 63:790-6. [ Links ]
15. Kleinberg I, Westbay G, Oral Malodor. Crit Rev Oral Biol Med 1990;1:247-59. [ Links ]
16. Miyazaki H, Sakao S, Katoh Y. Correlation between VSC and certain oral health measurements in the general population. J Periodontol. 1995; 66:679-84 [ Links ]
17. Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol 1990; 5:195-201 [ Links ]
18. Yoshida A, Yoshimura M, Ohara N, Yoshimura S, Nagashima S, Takehara T, Nakayama K. Hydrogen sulfide production from cysteine and homo-cysteine by periodontal and oral bacteria. Periodontology 2009; 80 (11): 1845-51. [ Links ]
19. Morita M, Wang HL. Association between oral malodor and adult periodontitis: a review, J Clin Periodontol 2001; 28: 813-9. [ Links ]
20. De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor, J Am Dent Assoc 1995; 126:1384-93. [ Links ]
21. Nakano Y, Yoshimura M. Koga T Correlation between oral malodor and periodontal bacteria. Microbes Infect 2002; 4: 679-83. [ Links ]
22. Rosenberg M.Clinical assessment of bad breath: current concepts. J Am Dent Assoc 1996;127: 475-82. [ Links ]
23. Awano S. Gohara K, Kurihara E, Ansai T, Takehara T The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int Dent J = [ Links ]
24. Messadi DV. Oral and nonoral sources of halitosis. J Calif Dent Assoc 1997; 25:127-31. [ Links ]
25. Spielman AI, Bivona P, Rifkin BR. Halitosis. A common oral problem. N Y State Dent J 1996;40:36-42. [ Links ]
26. Bosy, A. Oral malodor: philosophical and practical aspects. J Can Dent Assoc 1997; 63:196-201. [ Links ]
27. Preti G, Clark L, Cowart BJ, Feldman RS, Lowry LD, Weber E, Young IM . Non oral etiology of oral malodor and alterated chemosensation. . J Periodontol 1992; 63: 90-6. [ Links ]
28. Rashid M, Goldin R, Wright M. Drugs and the liver.Hosp Med. 2004;65(8):456-61. [ Links ]
29. Sherlock SThe spectrum of hepatotoxicity due to drugs. Lancet. 1986;23(8504):440-4. [ Links ]
30. Challenger F., Walshe J. M.,: Foetor hepaticus . Lancet 1955;1:1239-41 [ Links ]
31. Stuart C, Zieve L, Mahadevan V, Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J Lab Clin Med 1970; 75 (4): 628-35 [ Links ]
32. Canellakis ES, Tarver H. The metabolism of methyl mercaptan in the intact animal. Arch. Biochem , Biophys, 1953;42(2):446-55. [ Links ]
33. Ohigashi K, Tsunetoshi A, Ichiara K. The role of pyridoxal in methyl mercaptan formation, partial purification and resolution of methioninase. Med. J. Osaka Univ. 1951; 2:111, [ Links ]
34. Stuart C, Mahadevan V, Zieve L.Volatile fatty acids in the breath of patients with cirrhosis of the liver . J Lab Clin Med 1970;75(4):622-7, [ Links ]
35. Scott CA, Avellini C, Desinan L, Pirisi M, Ferraccioli GF, Bardus P, Fabris C,. et al. Chronic lymphocytic sialoadentis in HCV-related chronic liver disease::comparison with Sjogren's syndrome. His-topathology 1997; 30:41-8. [ Links ]
36. Ramos-Casals M, Garcia-Carrasco M, Cervera R, Rosas J, Trejo O, De la Red G, Sanchez-tapias JM et al. Hepatitis C infection mimicking Sjogren syndrome. A clinical and immunologic description of 35 cases. Medicine (Baltimore). 2001; 80(1): 1-8. [ Links ]
37. ToenzetichJ. Production and origin of oral malodor: a review of mechanism and methods of analysis. J Periodontol 1977;48:13-22. [ Links ]
38. Van Steeberghe D. Breath malodour, Curr Opin Periodontol 1997; 4:44 [ Links ]
 Correspondence:
Correspondence:
M Beushausen
Case Western Reserve University Department of Oral and Maxillofacial
Surgery. PGY 1
E-mail: max.beushausen@gmail.com













