Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.69 n.6 Johannesburg 2014
CASE BOOK
Oral medicine case book 61: Oral malignant melanoma
E MosalleumI; A AfroghehII; WP DreyerIII; JW SchneiderIV
IBDS. Division of Oral Pathology, Faculty of Dentistry, University of the Western Cape; National Health Laboratory Service, Tygerberg Hospital
IIBChD, MChD. Division of Oral Pathology, Faculty of Dentistry, University of the Western Cape; National Health Laboratory Service, Tygerberg Hospital
IIIBDS, HDipDent, PhD, FCD(SA)OMP. Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape; Professor Emeritus, Stellenbosch University
IVMBChB, MMed, FCPath(SA). Division of Anatomical Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University; National Health Laboratory Service, Tygerberg Hospital
CASE REPORT
A 45-year old male patient presented at the Oral and Maxillofacial Clinic, Tygerberg, with a pathological fracture of the left mandible following an extraction. The medical records of the patient revealed a history of multiple myeloma that was treated with Aredia (pamidronate disodium, an intravenous form of bisphosphonate), cyclophosphamide (an alkylating agent) and dexamethaxone (an anti-inflammatory and immunosuppressant drug). An orthopantomograph revealed osteonecrosis and pathological fracture of the left mandible, thought to be due to the earlier biphosphonate administration. The patient received conservative management for the osteonecrosis and was stable at the time of the publication, (19 months after the initial presentation with the pathological fracture).
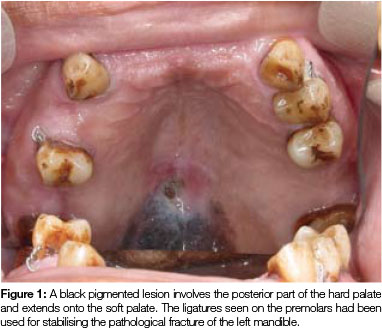
Clinical assessment during a routine follow-up visit, (31 months after treatment of multiple myeloma), revealed a flat, black pigmented lesion on the posterior part of the hard palate and involving the soft palate. The lesion had a slightly rough surface and measured 25 x 13mm in size (Figure 1). The patient had not noticed the lesion before and he had experienced neither pain nor discomfort in the area. Apart from a small polypoid lesion on the right buccal mucosa, no other oral or cutaneous lesions were present. An incisional biopsy of the palatal lesion was performed and the buccal polypoid lesion was removed at the same time.
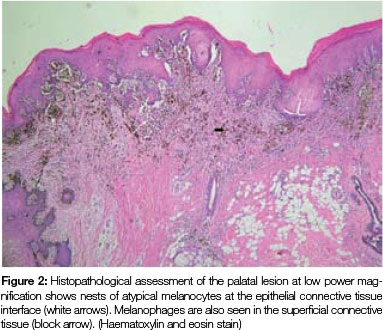
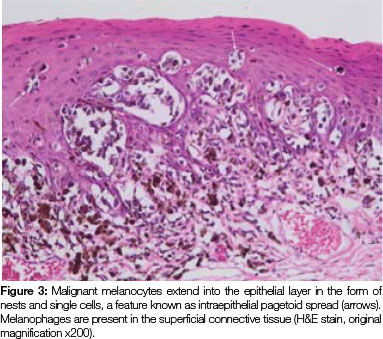
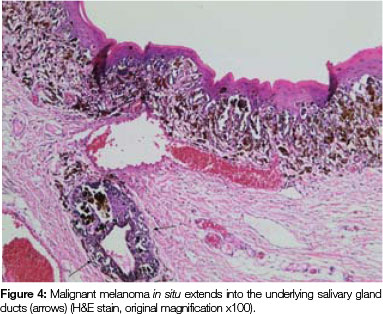
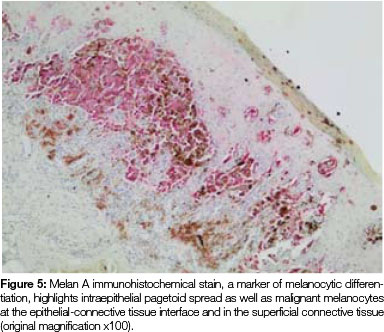
Both tissue samples were fixed in 10% buffered formalin and submitted to the National Health Laboratory Service (NHLS) for histological evaluation. Histopathological assessment of the lesion on the buccal mucosa disclosed a squamous papilloma whilst the incisional biopsy from the pigmented lesion showed a malignant melanoma in situ (Figure 2). The patient was subsequently referred for complete removal of the palatal lesion. Histopathological assessment of the excised specimen showed an invasive malignant melanoma with a prominent in situ component (see Figure 3-5). Invasive melanoma involved the anterior margin of the biopsy. However, anatomical constraints prevented more extensive removal of the melanoma and therefore the patient was scheduled for postoperative radiotherapy. Regular follow-up assessments of the patient revealed absence of recurrent melanoma or metastases 10 months postoperatively.
ACRONYM
OMM: Oral mucosal melanoma
DISCUSSION
Introduction
Melanomas are malignant neoplasms of melanocytes that derive from the neuroectoderm. Although the majority of melanomas involve the skin they can occur in any tissue containing melanocytes including the mucosa of the oral cavity, nasopharynx, eye, genital tract and anorectum. Oral mucosal melanoma (OMM) is a rare neoplasm that accounts for less than 0.5% of all oral malignancies and 0.2- 8% of all melanomas.1-12 Whilst melanocytes in the skin offer a protective function against ultraviolet light, their function in the oral mucosa is unknown and in the mucosal tissues they do not produce melanin under normal physiological states.1,2,5 Skin melanomas and OMM differ significantly in many respects. The mean age of onset of OMM is roughly a decade later than its cutaneous counterpart. The latter is a multi-factorial disease with evidence of genetic and ultraviolet light exposure as predisposing factors. As can be expected, mucosal melanomas are not influenced by sunlight and they also seem to have a different genetic background. For instance, cutaneous melanomas generally show alterations in the BRAF oncogene, (a gene that produces a protein that plays a role in regulating the signalling pathways for cell division and differentiation) whilst most OMM show an over-expression in c-KIT, a receptor tyrosine kinase that is a key regulator of growth, differentiation, migration and proliferation of melanocytes7, 10, 13 In addition, patients with OMM more frequently present with a more advanced stage of disease than skin lesions, due to the lack of early symptoms and relative inaccessibility. OMM therefore shows a poorer prognosis and limited 5-year survival rate in comparison to cutaneous melanoma.
In the present case, the OMM occurred some time after the patient received treatment for multiple myeloma, raising the question: is there any association between multiple myeloma and malignant melanoma? A recent report from a health-based social website states that in a group of 56,677 persons with multiple myeloma, 146 (or 0.26%) reported also having malignant melanoma.14 This number comprises only 0.63% of the 23,123 persons with malignant melanoma reported on the same website and is probably not statistically significant. Although melanoma may occur more frequently in patients with preceding lymphoma, a similar association has not been established for myeloma.15 It is theoretically possible that treatment with drugs affecting the immune status of a patient may stimulate an existing malignancy. However, this is purely speculation and will have to await more definitive analysis. The association with myeloma, reported in this patient, should thus be considered as purely coincidental.
Clinical presentation
The most common site for OMM is the hard palate, which accounts for 40% of all melanomas of the oral cavity. The maxillary gingiva and the alveolar ridge are the second most common sites, however, other oral sites can also be involved but OMM only rarely involves the mandibular gingiva.2, 5, 11, 12 The peak incidence is in the 5th to 7th decades of life with a predilection for males (M: F = 2:1).5 The lesion is usually asymptomatic and the diagnosis is thus often delayed except in patients who seek routine dental treatment or present for management of aesthetic problems.11, 12 Clinically, OMM varies from a macule to a nodule to a large exophytic mass with variable pigmentation. The border is usually irregular but is often well demarcated from the surrounding mucosa whilst the surface of the lesion may be wrinkled.12 The clinical differential diagnosis should at least include oral melanocytic naevi, melanoacanthoma and Kaposi sarcoma in addition to OMM.
The aetiological factors associated with OMMs are unknown and as may be expected, there is no association with sun exposure.2, 4 A history of a previous pigmented lesion, prior to the appearance of OMM, has been reported and contradictory reports have been published about the association between oral melanosis associated with tobacco usage, and oral malignant melanoma.5, 6, 12 Tobacco and formaldehyde exposure have been proposed as possible aetiological factors for OMM.12 Mucosal malignant melanoma is seen more frequently in some population groups from Asia, some parts of Africa and Japan.1
Histopathological features
The histomorphology of mucosal melanoma is variable and this is further complicated by non- pigmented and pigmented patterns. In malignant melanoma in situ, atypical melanocytes are distributed in a nested arrangement at the epithelium/connective tissue junction and with intraepithelial pagetoid spread, as seen in Figures 1 and 2 in the present case. Invasive OMM is characterised by invasion of malignant melanocytes into the underlying connective tissue, referred to as vertical growth. The malignant cells can vary in shape from spindled, epithelioid and plasmacytoid cells to small round blue cells. Confirmation of the melanocytic nature of the lesion can be done using immunohistochemical markers such as HMB45, Melan A or S100.7, 11, 12
OMM is a very aggressive disease usually presenting at an advanced stage. Usually no radial growth phase is observed in these lesions whilst a vertical (or nodular) growth phase occurs early thus leading to an advanced stage at presentation.2, 4 Unlike dermal melanomas, oral melanomas are classified histologically as in situ melanoma, invasive melanoma or combined in situ and invasive melanoma. Lesions that have uncertain histomorphological features of malignancy are termed atypical melanocytic hyperplasia.2
Molecular features of OMM
The molecular features of OMM are not as well established as those of cutaneous malignant melanoma largely due to the relative rarity of the former. Recent genetic studies identified mutations in DNA repair mechanisms that may be involved in the pathogenesis of OMM. These pathways include alterations in cyclin dependant kinase (CDKN2A) and mitogen-activated protein kinase (MAPK) pathways.4, 6 The former pathway is important in cell cycle regulation and the latter in cell growth and survival.4 As mentioned earlier, mutations in c-KIT (tyrosine receptor kinase) have been observed in 88% of oral melanomas, thus suggesting that the use of tyrosine receptor kinase inhibitors could be of therapeutic value in the management of head and neck mucosal melanomas.7, 10, 13
Management and prognosis
The prognosis of OMM is very poor and the 5-year survival rate of patients is in the order of 15% but this depends on the stage of the disease. Microstaging according to the guidelines for cutaneous melanoma, and in particular Clark levels and Breslow thickness, does not apply to OMM. The American Joint Committee on Cancer (AJCC) has a staging system for OMM which includes features of the primary tumour, lymph node status and metastases (TNM).16 Primary OMM confined to the mucosal epithelium is normally regarded as a T3 lesion to indicate its aggressive clinical course.2, 7 The poor prognosis of OMM, compared with cutaneous melanoma, relates to an advanced stage of development at the time of the patient's initial presentation.9 It should be stressed, however, that OMM is potentially curable if the tumour is diagnosed at an early stage when the lesion is still in its in situ phase and amenable to total surgical removal.12
Several clinical and pathological indicators have been shown to be associated with a poor prognosis in cutaneous melanomas. These include old age, male gender, specific sites such as the back, neck and acral skin and lymph node involvement. Likewise, the Breslow thickness and the presence of surface ulceration are histological indicators of poor overall survival and distant metastasis.7 Other histological factors of poor prognosis are perineural invasion, lymphovascular invasion and increased mitotic activity. On the contrary, the presence of tumour-associated lymphocytes is linked with a more favourable prognosis.8 Such prognostic factors, in cases of OMM and head and neck melanoma, are yet to be established and the cutaneous parameters for OMMs do not apply, largely due to the anatomic complexity of the head and neck region.8
OMM is treated by surgical excision, with clear mucosal margins, and the use of additional radiotherapy and chemotherapy in specific cases.11
CONCLUSION
Due to a lack of symptoms in the early stages of OMM, patients usually present late and early detection is thus uncommon. The importance of early detection and diagnosis cannot be overemphasised as it dramatically improves the prognosis. A high index of suspicion is needed when evaluating pigmented lesions of the oral cavity particularly in high-risk areas such as the gingiva and the palate. Oral health care practitioners need to approach all oral pigmented lesions with circumspection with due regard for the variable morphology and asymptomatic nature of OMM, which may prevent early detection and diagnosis. Moreover, information on the aetiopathogenesis of and the risk factors associated with mucosal melanoma is still sketchy and is open for future investigation.
Acknowledgements: The authors wish to thank Dr Mdalose for the clinical photograph.
Declaration: No conflict of interest declared.
References and *recommended reading
1. Turkmen A, Temel M, Bekerecioglu M. Primary mucosal malignant melanoma of the oral cavity. Eur J Plast Surg 2011, 34:325-9 [ Links ]
2. Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol 2000, 36: 152-69. [ Links ]
3. Kumar SK, Shuler CF, Sedghizadeh PP, Kalmar JR, Oral mucosal melanoma: epidemiology and pathobiology. J Cutan Pathol 2008, 35: 392-7 [ Links ]
4. Hsieh R, Nico MM, Coutinho-Camillo CM, Buim ME, Sangueza M, Lourengo SV. The CDKN2A and MAP kinase pathways: Molecular roads to primary oral mucosal melanoma. Am J Dermatopathol 2013, 35: 167- 75. [ Links ]
5. Lourengo SV, Bologna SB, Hsieh R, Sangueza M, Fernandes JD, Nico MM. Establishment and characterization of an oral mucosal melanoma cell line (MEMO) derived from a longstanding primary oral melanoma. Am J Dermatopathol 2013, 35: 248-51. [ Links ]
6. Garzino-Demo P, Fasolis M, Maggiore GM, Pagano M, Berrone S. Oral mucosal melanoma: a series of case reports. J Craniomaxillofac Surg. 2004, 32: 251-7. [ Links ]
7. Kerr, EH, Hameed O, Lewis JS, Bartolucci AA, Wang D, Said-Al-Naief N. Head and neck mucosal malignant melanoma: Clinico-pathologic correlation with contemporary review of prognostic indicators. Int J Surg Pathol 2012, 20: 37- 46. [ Links ]
8. Homsi J, Kashani-Sabet M, Messina JL, Daud A. Cutaneous melanoma: prognostic factors. Cancer Control. 2005,12: 223- 9. [ Links ]
9. González-Garcia R, Naval-Gías L, Martos PL, Nam-Cha SH, Rodríguez-Campo FJ, Muñoz-Guerra MF, Sastre-Pérez J. Melanoma of the oral mucosa. Clinical cases and review of the literature. Med Oral Patol Cir Bucal. 2005, 10: 264- 71. [ Links ]
10. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006, 24: 4340- 6. [ Links ]
11. McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma Oral Oncology 2008, 44: 1039- 46. [ Links ]
12. Padhye A, D'Souza J,. Oral malignant melanoma: A silent killer?. Journal of Indian Soc Periodontol 2011, 15: 425- 8. [ Links ]
13. *Seetharamu N, Ott PA, Pavlick DC. Mucosal melanomas: A case-based review of the literature. The Oncologist 2010, 15:772-81. [ Links ]
14. http://www.ehealthme.com/cs/multiple+myeloma/malignant+melanoma [ Links ]
15. Brewer JD, Habermann TM, Shanafelt TD. Lymphoma-associated skin cancer: incidence, natural history, and clinical management. Int J Dermatol 2014, 53:267-74. [ Links ]
16. Brandwein-Gensler M, Smith RV. Prognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging system. Head Neck Pathol 2010, 4:53-61. [ Links ]
 Correspondence:
Correspondence:
WP Dreyer
PO Box 1285, Sedgefield, 6573
E-mail wpd@sun.ac.za













