Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Bothalia - African Biodiversity & Conservation
versión On-line ISSN 2311-9284
versión impresa ISSN 0006-8241
Bothalia (Online) vol.54 no.1 Pretoria 2024
http://dx.doi.org/10.38201/btha.abc.v54.5
ORIGINAL RESEARCH
Comparing the effectiveness of pitfall traps and active sampling methods for ants and spiders in a Chromolaena odorata invaded site
Vanessa L. LauchandeI; Sinenhlahla P. MntamboI, II; Zabentungwa T. HlongwaneIII; Thinandavha C. MunyaiI
ISchool of Life Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville 3209 South Africa
IISchool of Biology and Environmental Sciences' University of Mpumalanga, Mbombela 1200, South Africa
IIISchool of Life Sciences, University of KwaZulu-Natal, Private Bag X541001, Durban 4000, South Africa
ABSTRACT
BACKGROUND: Active and passive arthropod sampling techniques have their specific limitations. Pitfall trapping is a commonly used passive sampling method, and bush beating, aerial hand collection above the knee, aerial hand collection below the knee cryptic and non-cryptic are widely used active sampling techniques.
OBJECTIVE AND METHOD: Pitfall traps and four active sampling techniques were used in a Chromolaena odorata invaded site to compare the methods used in sampling arthropods in Buffelsdraai Conservancy outside the city of Durban, South Africa.
RESULTS: Pitfall traps were the most efficient and the most effective sampling technique with high species richness for both the ant (78%) and spider (76%) samples. One explanation for these differences could be the longer sampling time for passive sampling compared to active sampling.
CONCLUSION: Compared to the subjective identification of species by collectors in active techniques, the non-selective capturing of species by pitfall traps improves its efficiency. The fewest taxa and individuals were collected by aerial hand collection techniques but these techniques are recommended to supplement pitfall traps. The combination of methods allows for the adequate sampling of the various strata found in vegetatively complex sites. An investigation into the possible use of canopy techniques in C. odorata sites would be beneficial, as it considers the various vegetation strata when sampling for biodiversity.
Keywords: Aerial hand collection techniques; pitfall traps; bush beating; biodiversity; ant; spider; Chromolaena odorata.
Introduction
Arthropods are found in all ecosystems on Earth and make up most of the biodiversity. They are known to contribute to ecosystem services and maintain the environment (Maleque et al. 2006). Therefore, the shift in their presence and population may be an efficient indicator of changes in the ecosystem (Ozanne 2005). Their vast abundance hinders the quantification of arthropods and has resulted in the development of various sampling techniques (Lowman et al. 1996). The lack of standardisation has led to numerous interpretations of biodiversity estimations for diverse ecosystems (Moir et al. 2005).
Among arthropods, ants are a well-known keystone taxon, highly diverse, and abundant. Their resilience makes them highly sought after when attempting to assess the biodiversity of many different ecosystems (Groc et al. 2007). Pitfall trapping is generally used when sampling ants, except for more vegetatively complex ecosystems where suitable methods such as the Winkler or Malaise sampling method are recommended (Parr & Chown 2001; Sheikh et al. 2018). In contrast, Majer (1997) recommended conducting both hand collection and pitfall traps to get a more complete estimate of ant diversity within complex ecosystems.
Spiders form a highly diverse predator group that globally impacts many tropical ecosystems' functionalities (Kapoor 2006). The relative ease with which spiders are captured has led to the development and use of various cost-effective sampling methods. The limit to these methods is that they generally concentrate on a specific assemblage of arthropods, resulting in a misrepresentation of the community (Green 1999).
Ozanne (2005) found that using various sampling methods simultaneously ensures a more comprehensive assessment. For example, pitfall traps collect ground and leaf litter-dwelling arthropods, while aerial techniques collect a more diverse range of arthropods from different vegetative strata (Malumbres-Olarte et al. 2017). As these invertebrates are sampled to represent the biodiversity within an ecosystem, conducting assessments on diversity changes in invaded forests is of high importance (Muelelwa et al. 2010), but rarely can experiments be performed in ideal conditions, as time and financial constrictions may compel researchers to use a single sampling method (Parr & Chown 2001).
Many restoration projects are implemented on invaded sites to increase biodiversity levels. Efficient biodiversity assessments need to be conducted to represent the change in diversity caused by these invasive species (Muelelwa et al. 2010). As Chromolaena odorata is a widespread invasive plant species in South Africa, investigating the impacts it might have on the biodiversity of the invaded ecosystem is of high priority for many ecological researchers (Rejmánek & Richardson 2013).
A series of sugarcane farms located in Buffelsdraai outside the city of Durban in South Africa, originally a scarp forest, are being restored. This has the potential to restore the overall biodiversity that was previously lost. Many cleared forests in Africa and Asia are vulnerable to invasion by C. odorata. Therefore, if not biologically controlled and monitored, it hinders many future restoration projects. Reforestation programmes must be monitored to ensure they are accomplishing their intended goals, which are often to restore biodiversity loss (Kanowski et al. 2008). Consistent monitoring of reforested landscapes gauges the success of restoration long after the restoration has been completed (Gerlach et al. 2013).
Of equal importance is a need to quantify and standardise the various methods used to collect biodiversity data for monitoring the success of these monitoring programmes. For arthropods occurring in complex habitat types like forests or alien plant invaded habitats, various sampling methods are available to estimate their diversity in each ecosystem (Moir et al. 2005). These methods tend to be biased depending on the time spent and the number of traps used for the specific technique and externally biased by factors such as the target species and the sampling environment (Mc-Cravy 2018). These methods include both active and passive sampling methods as employed by Muelelwa et al. (2010) and Malumbres-Olarte et al. (2017). Malumbres-Olarte et al. (2017) found that combined methods were necessary to ensure that the spider diversity was adequately represented within a vegetatively complex area such as forests.
The current study, therefore, sampled ants and spiders using active and passive methods in sites invaded by C. odorata to test their sampling efficiency within vegetatively complex habitats. This study's objectives were to: (i) determine the difference in ant and spider diversity sampled between passive and active sampling methods; and (ii) determine if the ant and spider composition varies between sampling methods. This study hypothesised a difference in diversity and assemblage composition for ants and spiders sampled using techniques employed. We further predicted that active sampling would have a lower species diversity than passive sampling because the longer sampling time increased the capturing potential. We also predicted that there will be a unique assemblage composition associated with active sampling techniques. And lastly, that the individual selection of rare species by aerial hand-collection techniques will reduce the similarity in species composition.
Methods and materials
Study area
The study was conducted in the Buffelsdraai Conservancy (29°37'50.17188" S, 30°59'0.77352" E), approximately 25 km north of Durban in KwaZulu-Natal, South Africa. Historically a forested area, 750 ha of the conservancy was cleared for sugarcane production over a 100 years ago. The cultivation of sugarcane was then halted and cleared in 2008, to enforce the Buffelsdraai Landfill Site Community Reforestation Project initiated by the eThekwini Municipality. The primary reason for this was initially to offset the excessive emission of greenhouse gases during the FIFA 2010 World CupTM in Durban. Additionally, it will be used to mitigate any carbon emission from the nearby landfill site and encourage surrounding communities to grow indigenous tree seedlings to produce goods and food. The study area is located 200-325 m.a.s.l.; this region is characterised by a hot and wet climate in summer, with a cool and dry winter, receiving precipitation of approximately 766 mm in summer, and a mean temperature of 22.2°C in winter to 27.4°C in summer.
The area falls within the KwaZulu-Natal Coastal Belt vegetation type, which is dominated by grasslands and subtropical trees. The cleared sugar cane farm is infested by the invasive species C. odorata. The current study was conducted along treatments/habitat types with different gradients of C. odorata invasion (high, moderate and none). The gradients of invasion were determined relative to the visible density of C. odorata found in the sampling site. High invasion included dense coverage of C. odorata, moderate had a scattering of C. odorata coverage, and none had no C. odorata presence. All these sites/habitats were replicated four times. Also, two habitats representing C. odorata cleared and uncleared, each replicated five times, were also sampled.
Ant and spider sampling methods
Most sampling techniques used have been divided into passive and active sampling techniques, depending on the involvement of the collector (Grootaert et al. 2010). The primary passive sampling technique routinely used is pitfall trapping. It involves the focal taxa movements toward the trap (Grootaert et al. 2010; Zou et al. 2012). This cost-effective sampling technique requires less maintenance and eliminates the researcher's subjective bias (Sheikh et al. 2018). In contrast, active sampling methods involve the diligent searching and collecting of arthropods by the researcher, leading to subjective biases (Grootaert et al. 2010; Zou et al. 2012).
Three widely used active techniques are: (i) sweep netting, which involves swinging a net through vegetation, mostly in grassland; (ii) aerial hand collection, which requires the researcher to collect the visible arthropods in each area (Lowman et al. 1996; Moir et al. 2005); and (iii) vegetative beating, which requires the collection of fallen arthropods with a tray from a shaken tree (Ozanne 2005). These methods tend to collect specific arthropod groups and their suitability varies with ecosystem-type.
Pitfall trapping (passive sampling method)
Pitfall traps are efficient for capturing ground-dwelling ant and spider species. Each sampling grid (2 x 5 grids) had ten plastic pitfall traps (± 56 mm diameter) dug into the ground. In each sampling grid, pitfall traps were 10 m apart (total length of the grid, therefore, equal to 50 m). The pitfall traps were half-filled with propylene glycol, which is not an ant attractant or repellent (Munyai & Foord 2011). The pitfall traps were left open for five days at each site. This duration has previously been proven to avoid both over and under-sampling of ant populations (Munyai & Foord 2015). Ants and spiders found in the traps were separated from other invertebrates and stored in 70% ethanol.
Active search methods
Active search sampling methods for ants and spiders included vegetation beating (BB), aerial hand collection above the knee (AHC), aerial hand collection below the knee cryptic (AHC cryptic) and aerial hand collection below the knee noticeable or non-cryptic (AHC OBV). Since densely vegetative ecosystems obstruct the sampling ability to sweep netting, it is commonly replaced with beating in dense vegetation.
For the active search methods, the 50 m transect was divided into three intervals; 0-25 m, 25- 35 m, and 35-50 m. Specimens collected per interval were stored on one vial half-filled with 70% alcohol. Four people carried out active searches. Each person searched for 15 minutes simultaneously per plot/sampling transect. Similar to Robertson et al. (2011), the same person conducted each active search method to try to standardise collector bias.
Ant and spider identification
Ant specimens were identified either to species level using voucher specimens in the School of Life Science, University of KwaZulu-Natal (UKZN) in Pietermaritzburg campus or to genus level using Fisher and Bolton (2016) and then assigned to morphospecies by the last author. A voucher collection with representative specimens is currently placed at the School of Life Sciences at UKZN and Iziko Museum of Cape Town. A.S. Dippenaar-Schoeman at the Agricultural Research Council (ARC) identified the spider specimens to species levels where possible or otherwise genus and then morphospecies. All specimens are housed at ARC in Pretoria.
Statistical analysis
To analyse the collected data, a constant of one was added to the count abundance to ensure that all zero values are logged and analysed. A one-way ANOVA was used on R (R Core Development Team, 2017) to compare active and passive sampling techniques in sampling ant and spider species. The spider and ant data were log transformed to ensure that the assumption of normality was met. The Welch one-way test was run on the ant species collected to test for the difference between passive and active sampling techniques. An alternative one-way ANOVA was conducted on the spider data as they continued to violate normality after being transformed. The Kruskal-Wallis rank sum test was used to test the difference in spider species collected between passive and active sampling methods.
To determine the differences in arthropod diversity within different sampling methods in the study site, the collected data were analysed using diversity indices Simpson's Diversity Index (D), Shannon-Wiener Diversity Index (H'), and Evenness (J') in R (R Core Development Team 2017).
A coverage estimator was used to assess sample completeness (coverage-based rarefaction extrapolation) described by (Chao & Jost 2012). A sample completeness analysis was conducted using iNEXT (Chao et al. 2016).
Using the analysis of similarity (ANOSIM) from the Primer 6+ software package (Clarke & Green 1988), the difference in ant and spider assemblage found by the active and passive sampling strategies were compared. The Global R generated represents the closeness to the compared assemblages, when the significance value is closer to one there is more of a difference (Clarke & Gorley 2001). To adequately represent this significance, estimation of difference was established, the significance of R > 0.75 is clearly separated, R > 0.5 partially overlaps but is different, and R < 0.25 mostly overlaps (Hamer & Slotow 201 7). A Non-metric Multi-Dimensional Scaling (NMDS) was conducted using the Global R-value generated to graphically represent these findings, where points with closer distances have more similar assemblages (Patrick et al. 2012).
Results
Ant and spider composition as sampled by various sampling techniques
The passive and active sampling techniques collected 52 222 ants, representing six subfamilies, 24 genera, and 61 species (Table 1A). The collected samples adequately represented the ant community, as the coverage of the sample size was above 0.96 (Figure 1A, Table 2). Formicinae was the most abundant (90.12%) subfamily within the ant specimens, represented by six genera and 14 species (Table 1A), followed by the three subfamilies, each with an abundance below ten percent (Table 1A). The least abundant subfamily was Dorylinae (0.004%), represented by a single genus Aenictus (Table 1).
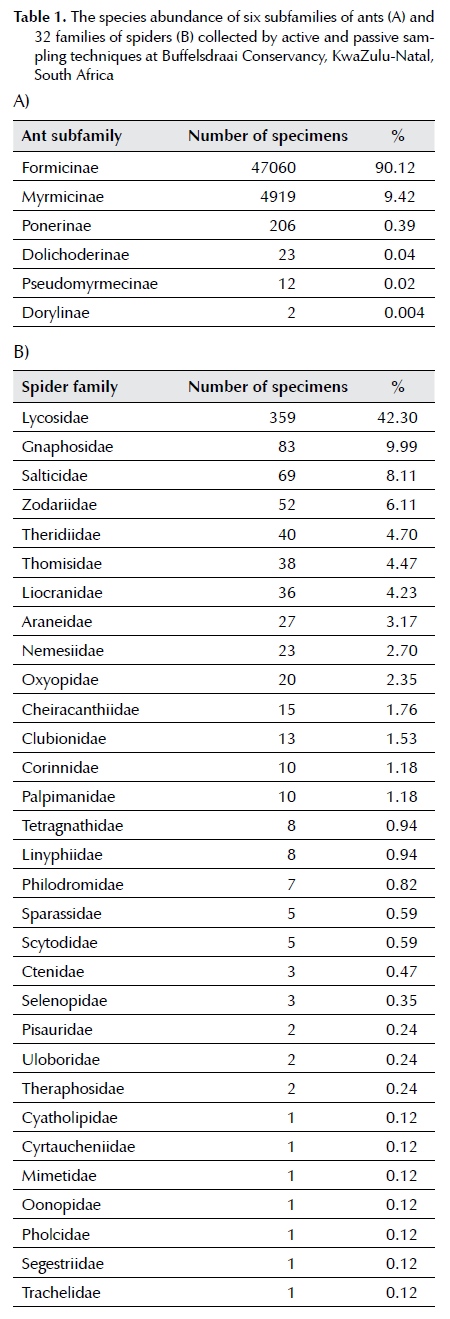
A total of 851 spiders, representing 32 families, 82 genera and 132 species, were collected using both passive and active sampling techniques (Table 1b). The collected samples were an inadequate representative of the spider community, as the sample size coverage was below 0.5, except for the pitfall trap, which was above 0.97 (Figure 1B, Table 3). Lycosidae was the most abundant (42.30%) and diverse family with six genera and nine species found (Table 1B). Thirteen families each yielded an abundance of more than one percent (Table 1B).
Seventeen families each yielded an abundance below 1% (Table 1B), of which seven families yielded the lowest abundance (0.12%) and each represented single genera and species (Table 1B). Overall, pitfall trapping was the most successful technique (Table 2 and 3). The sampling technique that collected the fewest individuals of both ants and spiders was aerial hand collection below knee cryptic (Table 2 and 3).
Ant and spider species diversity as sampled by various sampling techniques
Pitfall traps yielded the highest diversity but the lowest variation between taxa (Table 2 and 3). Bush beating was most successful for spiders and sampled the highest diversity (Table 2). Furthermore, pitfall traps yielded the second-highest diversity but the lowest variation for both taxa. For both the ants and spiders, the least diversity but the highest variation in taxa was collected by aerial hand collection below knee cryptic (Table 2 and 3).
There was a significant difference (F-value = 91.72; d.f.n = 4; d.f.d = 51.25; n = 16; Total = 30.956 ± 158.186; p < 0.0001) between ant species collected by passive and active sampling techniques. Pitfall traps contributed the highest species richness (39.190 ± 178.320). In comparison, aerial hand collection, below the knee cryptic, contributed the least ant richness (1.405 ± 0.627). The passive sampling technique collected a smaller number of ant species within the C. odorata invaded sites (Figure 2).
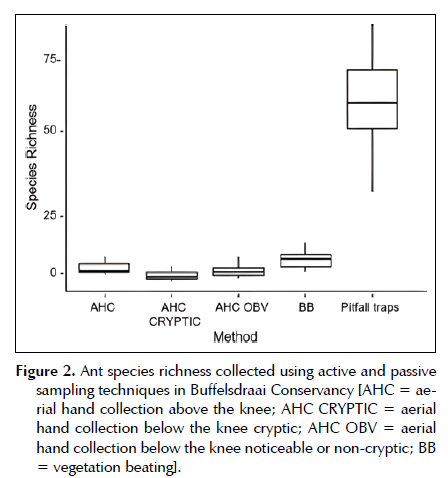
Within the group, the comparison between AHC and AHC OBV was found to not be significantly different (p = 0.299). There was a considerable difference in the means between the rest of the compared sampling techniques, contributing to the variation in richness found as all pitfall comparisons had high significance (p < 0.0001). The latter can be inferred that it influenced the different ant species richness (Table 4).
There was a significant difference [H (2) = 57.59, df = 4, mean rank: Pitfall trap = 23.908; BB = 4.681; AHC = 1.545; AHC OBV = 1.091; AHC CRYPTIC = 0.636, Total = (1.255 ± 1.215), p < 0.0001], between spider species collected by passive and active sampling techniques. Pitfall traps once again contributed the most spider richness (1.283 ± 1.362). Aerial hand collection, below the knee non-cryptic, contributed the least to spider richness (1.25 ± 0.442). The passive sampling technique collected the highest number of spider species sampled within the C. odorata invaded sites (Figure 3).
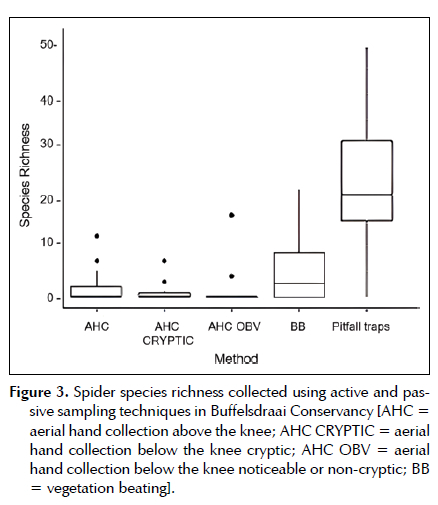
There was a significant difference between bush beating and aerial hand collection above the knee non-cryptic (Table 5). The latter sampling methods seem to have low to no contribution to the variation in mean richness. Pitfall trap comparisons had high significance (p < 0.0001). Therefore, it was surmised that pitfall traps had the most influence on the differing spider richness (Table 5).
Ant and spider assemblage composition collected by passive and active sampling techniques
The NMDS illustrates a similarity in ant assemblages within passive sampling techniques and similarity within active sampling. Still, there was a distinct separation in ant composition between passive and active sampling techniques (Figure 4A). The ANOSIM also revealed that these are a separated (Global R = 0.945; p = 0.001) assemblage. The NMDS illustrates a similarity in spider's assemblage between sampling techniques, with separation occurring between AHC and AHC CRYPTIC techniques (Figure 4B). The ANOSIM also revealed that there is a partial overlap but a different (Global R = 0.366; p = 0.001) assemblage.
Discussion
In the present study, the ant and spider passive technique samples were comprehensive, with more than 92% sample completeness (Figure 1A and 1B; Table 2 and 3). Therefore, the pitfall trapping is reported as a sampling technique that have represented the sufficient collection of ant and spider diversities found within the sampling site. This is comparable to a study by Muelelwa et al. (2010), who assessed the community of spiders in various woodland, bushveld and forest habitats. The latter study was conducted in the Blouberg Nature Reserve and Western Soutpansberg Conservancy in Limpopo, South Africa. Notably, for ant samples, all active sampling methods were comprehensive with more than 90% of sampling completeness (Table 2). However, spider samples had low sample completeness between 0 and 60% (Table 3). The low percentages suggest that the active techniques data was a poor representation of the site's spider community. However, additional sampling to meet the estimated richness is unfeasible, and the current sampling completeness is then conserved (King & Porter 2005).
In a spider study, Azevedo et al. (2014) argued that low completeness was explained by the high proportion of rare species found in forests, which could be found in the sampled invaded sites. Similar to Cardoso (2009), the expected difference in diversity between passive and active sampling was present, with higher estimated taxonomic diversity in arthropods collected by pitfalls (Table 2 and 3). Within active techniques, the bush beating had the highest estimated diversity compared to hand collection (Table 2 and 3). Tourinho et al. (2018) reported that spiders are generally arboreal and occupy branches of various tree species. Bush beating then dislodges the spider individuals and subsequently, they are collected in the beating sheet. A different study, McCravy (2018), emphasised that bush beating collects a higher proportion of arthropods and that a higher probability of new species is collected.
As expected, the relative distribution (evenness) of spider species was greater in aerial hand collection, cryptic and non-cryptic (Table 3). Whereas the ants higher relative distribution was demonstrated in aerial hand collection below the knee cryptic (Table 2). The abundance of arthropods collected by the aerial hand collection technique is limited in abundance but is methodical in collecting species of specific microhabitats (S0rensen et al. 2002). Like Privet et al. (2020), the individual collection of present arthropods led to a greater variety of species, whether they are mobile, sedentary or hunting, relative to pitfall traps, which were limited to mobile species (Missa et al. 2009). Additionally, the placement of pitfall traps limits the evenness. Pitfall traps located near colony nests increases the collection of individuals from a single species (Grootaert et al. 2010; Sheikh et al. 2018).
Pitfall traps had the highest significant effect on the mean variation of species richness observed (Figure 2 and 3). It also collected higher abundances of species. However, the evenness demonstrates that this abundance is biased toward specific species. They were the most efficient, as this technique collected 78% of the ant species and 76% of the spider species of all methods used in the current study.
The sampling time could have caused the difference between the efficiency of the two techniques. For example, a five-day capture time of pitfall traps allows for the incidental capturing of nocturnal, diurnal and colonising ant and spider species not easily identified by collectors during active sampling (Prasifka et al. 2007). The longer sampling time also helps capture less abundant and less active species (Bali et al. 2019). Moir (2005) reported bush beating as the second most fruitful sampling technique used when sampling ants and spiders (Table 2 and 3). The latter study also observed that bush beating was time efficient but biased against small-bodied taxa.
Like Nsengimana et al. (2017), the current study observed the lowest effect on the mean variation of species richness in aerial hand collection, below the knee cryptic, and non-cryptic active sampling techniques (Figure 2 and 3). The major disadvantages mentioned by several studies are linked to the ant and spider size as larger individuals are easier to identify (Privet et al. 2020).
Additionally, the reduced time limits collecting potential, and the collector's experience restricts the potential for identification, as it is time-consuming and may lead to the collector's fatigue (Berthold et al. 1999; S0rensen et al. 2002; Tuf 2015; Nsengimana et al. 2017). Lastly, active sampling causes a disturbance within the habitat. This disturbance causes an underrepresentation of numbers as many species hide to avoid the collectors (Bali et al. 2019).
Corresponding with Silva et al. (2013), pitfall traps predominately sampled ground-dwelling ants (belonging to the subfamily Formicinae), as this technique is suitable for capturing arthropods actively present in the soil (Table 1A and 2). Pitfalls were also noted to sample a greater number of ant species compared to that of active sampling methods, as the small stature of ants make it harder to identify (Table 2). Similar to Siewers et al. (2014), the current study found that pitfall traps collected a higher number of large-bodied spider species (belonging to the family Lycosidae) compared to small-bodied spider species (belonging to the family Pholcidae) (Table 1b and 3). The difference in size between captured spiders is attributed to the ability of small spiders to escape pitfall traps (Bali et al. 2019). Furthermore, as pitfall traps are frequently used to collect ground-dwelling arthropods, this technique's efficiency in collecting predominately arboreal spiders would be low (McCravy 2018).
The big-bodied epigean spiders (species from the family Lycosidae and Gnaphosidae) were most sampled by pitfall traps (Majer 1997; McCravy 2018; Bali et al. 2019). Based on the NMDS, the ant assemblage indicates a high similarity in ant species collected for each pitfall trap replicate. This assemblage was distinctly separated from active sampling techniques (Figure 4A). As observed, there was a variation within the active sampling techniques. Thayer and Werner (2007) stated that hand collection allows for collecting rare species in microhabitats. However, the success of such sampling will depend on the collector's expertise in identifying potentially rare taxa and the ability to collect them. There was a similar composition between bush beating and aerial hand collection above the knee while aerial hand collection below the knee cryptic and non-cryptic had a similar ant composition. Contrary to this, spider assemblage indicated that mostly all active and passive replicates overlapped with a distinct separation in a single replicate of hand collection above and below cryptic techniques (Figure 4B).
Mgobozi et al. (2008) observed low richness in invaded sites. The current study also reported similar results where low levels of species richness were found for pitfall samples collected in uncleared, high and medium-invaded sites. The likely explanation for the reduction in richness might be the dense vegetation that hindered the trapping of ground-dwelling arthropods. Secondly, plant diversity loss reduced the microhabitats' complexity (Mgobozi et al. 2008; Malumbres-Olarte et al. 2013).
The current study found that pitfall trapping is the most efficient method for sampling ant and spider populations. However, compared to the sampling techniques used, the results present an incomplete representation of ant and spider diversity. The evenness of pitfall traps may be improved by including baiting, as it allows for the collection of individuals from various species attracted to the bait (Sheikh et al. 2018). It would be beneficial to use aerial hand-collection techniques to supplement pitfall traps (Lowman et al. 1996). To improve the study, canopy sampling techniques (for example fogging) must be considered as C. odorata grows about six meters tall, encompassing various arboreal species (Malumbres-Olarte et al. 2017).
Conclusion
In conclusion, pitfall traps (as employed here) collected more arthropods from more taxa than either beating or sweep-netting within the C. odorata invaded sites of the Buffelsdraai Conservancy. This is likely partly due to the longer time pitfall traps were deployed for compared to the time allowed for the active sampling conducted by the collectors. However, all the sampling techniques have biases. Pitfall traps captured various arthropods while the active sampling techniques were biased toward large-bodied and actively-hunting arthropods. Furthermore, the relative disturbance caused by active sampling reduces the collecting potential and reduces efficiency.
Consequently, biodiversity assessment studies should employ both pitfall traps and active sampling techniques. Specifically, a combination of pitfall traps and hand collection is recommended within forested or similar regions and requires canopy sampling to sample biodiversity adequately.
Acknowledgements
Nokubonga Thabethe, Lindiwe Khoza, Nomathamsanqa Mkhize, Concilia Mukanga, Thandeka Mahlobo and Sbongiseni Xolo are thanked for their help in the field. Lastly, thanks to eThekwini Municipality for allowing us to work at their conservancy. Special thanks to Fatima Alli for helping to identify potential sites for sampling and Prof A.S. Dippenaar-Schoeman at the Agricultural Research Council (ARC) for identifying the spiders.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
T.C.M. (University of KwaZulu-Natal) designed and conceptualised the study; S.PM. (University of Mpumalanga) and T.C.M. collected the data; T.C.M. led the curation and identification of arthropods. V.L.L. (University of KwaZulu-Natal) analysed and led the writing (as part of her BSc honours dissertation), under the supervision of T.C.M. Z.T.H. (University of KwaZulu-Natal) formatted and presented the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Ethical considerations
Permission to sample ants and spiders in the study site (Buffelsdraai Conservancy) was approved by the Environmental Planning & Climate Protection Department of the eThekwini.
Funding
The Centre for Invasion Biology and South Africa's National Research Foundation Grant number 114416 provided funding.
References
Azevedo, G.H.F., Faleiro, B.T., Magalhães, I.L.F., Benedetti, A.R., Oliveira, U., Pena-Barbosa, J.PP & Santos, A.J., 2014, 'Effectiveness of sampling methods and further sampling for accessing spider diversity: A case study in a Brazilian Atlantic rainforest fragment', Insect Conservation and Diversity, 7, 381-391, https://doi.org/10.1111/icad.12061. [ Links ]
Bali, L., Andrési, D., Tuba, K. & Szinetár, C., 2019, 'Comparing pitfall trapping and suction sampling data collection for ground-dwelling spiders in artificial forest gaps', Arachnology Letters, 58, 23-28, https://doi.org/10.30963/aramit5808. [ Links ]
Berthold, A., Bruckner, A. & Kampichler, C., 1999, 'Improved quantification of active soil microfauna by counting crew', Journal of Biology and Fertility of Soils, 28, 352-355, https://link.springer.com/article/10.1007/s003740050503. [ Links ]
Cardoso, P, 2009, 'Standardization and optimization of arthropod inventories-the case of Iberian spiders', Biodiversity and Conservation, 18, 3949-3962, https://doi.org/10.1007/s10531-009-9690-7. [ Links ]
Chao, A., Chiu, C. & Jost, L., 2016, 'Statistical challenges of evaluating diversity patterns across environmental gradients in mega-diverse communities', Journal of Vegetation Science, 27, 437-438, https://doi.org/10.1111/jvs.12420. [ Links ]
Choa, A. & Jost, L., 2012, 'Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size', Ecology, 93, 2533-2547, https://doi.org/10.1007/s003740050503. [ Links ]
Clarke, K.R. & Gorley, R.N., 2001, Primer v5: user manual/ tutorial. Primer-E Ltd, Plymouth. [ Links ]
Clarke, K.R. & Green, R.H., 1988, 'Statistical design and analysis for a biological effects study', Marine Ecology Progress Series, 46, 213-226, https://www.jstor.org/stable/24827586. [ Links ]
Fisher, B.L. & Bolton, B., 2016, Ants of Africa and Madagascar: a guide to the genera, University of California Press, California, USA, https://doi.org/10.1525/9780520962996. [ Links ]
Gerlach, J., Samways, M. & Pryke, J., 2013, 'Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups', Journal of Insect Conservation, 17, 831-850. [ Links ]
Green, J., 1999, 'Sampling method and time determines composition of spider collections', The Journal of Arachnology, 27, 176-182, https://www.jstor.org/stable/3705982. [ Links ]
Groc, S., Delabie, J.H.C., Céréghino, R., Orivel, J., Jaladeau, F., Grangier, J., Mariano, C.S.F. & Dejean, A., 2007, 'Ant diversity in the 'Grand Causses' (Aveyron, France): In search of sampling methods adapted to temperate climates', Comptes Rendus Biologies, 330, 913-922, https://doi.org/10.1016/j.crvi.2007.08.012. [ Links ]
Grootaert, P., Pollet, M., Dekoninck, W. & Van Achterberg, C., 2010, 'Chapter 15 - Sampling insects: general techniques, strategies, and remarks', ABC Taxa, 8, 377-399. [ Links ]
Hamer, M.L. & Slotow, R., 2017, 'A conservation assessment of the terrestrial invertebrate fauna of Mkambati Nature Reserve in the Pondoland Centre of Endemism', Koedoe, 59, 1-12, https://hdl.handle.net/10520/EJC-7311b112f. [ Links ]
Kanowski, J., Catterall, C.P & Harrison, D.A., 2008, 'Monitoring the outcomes of reforestation for biodiversity conservation', in N.E. Stork & S.M. Turton (eds.), Living in a dynamic tropical forest landscape, pp. 526-536, Wiley-Blackwell, Oxford, UK, https://doi.org/10.1002/9781444300321.ch42. [ Links ]
Kapoor, V., 2006, 'An assessment of spider sampling methods in tropical rainforest fragments of the Anamalai Hills, Western Ghats, India', Zoos Print Journals, 21, 2483-2488, https://doi.org/10.11609/JoTT.ZPJ.1520.2483-8. [ Links ]
King, J.R. & Porter, S.D., 2005, 'Evaluation of sampling methods and species richness estimators for ants in upland ecosystems in Florida', Environmental Entomology, 34, 1566-1578, https://doi.org/10.1603/0046-225X-34.6.1566. [ Links ]
Lowman, M.D., Kitching, R.L. & Carruthers, G., 1996, 'Arthropod sampling in Australian subtropical rain forests - how accurate are some of the more common techniques?', Selbyana, 17, 36-42, https://www.jstor.org/stable/41759922. [ Links ]
Majer, J.D., 1997, 'The use of pitfall traps for sampling ants - a critique', Memoirs of the Museum of Victoria, 56, 323-329, https://doi.org/10.24199/j.mmv.1997.56.20. [ Links ]
Maleque, M.A., Ishii, H.T. & Maeto, K., 2006, 'The use of arthropods as indicators of ecosystem integrity in forest management', Journal of Forestry, 104, 113-117, https://doi.org/10.1093/jof/104.3.113. [ Links ]
Malumbres-Olarte, J., Scharff, N., Pape, T., Coddington, J.A. & Cardoso, P., 2017, 'Gauging megadiversity with optimized and standardized sampling protocols: A case for tropical forest spiders', Ecology and Evolution, 7, 494-506, https://doi.org/10.1002/ece3.2626. [ Links ]
Malumbres-Olarte, J., Vink, C.J., Ross, J.G., Cruickshank, R.H. & Paterson, A.M., 2013, 'The role of habitat complexity on spider communities in native alpine grasslands of New Zealand', Insect Conservation and Diversity, 6, 124-134, https://doi.org/10.1111/j.1752-4598.2012.00195.x. [ Links ]
McCravy, K.W., 2018, 'A review of sampling and monitoring methods for beneficial arthropods in agroecosystems', Insects, 9, 1-27, https://doi.org/10.3390/insects9040170. [ Links ]
Mgobozi, M.R., Somers, M.J. & Dippenaar-Schoeman, A.S., 2008, 'Spider responses to alien plant invasion: the effect of short- and long-term Chromolaena odorata invasion and management', Journal of Applied Ecology, 45, 1189-1197, https://doi.org/10.1111/j.1365-2664.2008.01486.x. [ Links ]
Missa, O., Basset, Y., Alonso, A., Miller, S.E., Curletti, G., De Meyer, M., Eardley, C., Mansell, M.W. & Wagner, T., 2009, 'Monitoring arthropods in a tropical landscape: relative effects of sampling methods and habitat types on trap catches', Journal of Insect Conservation 13, 103-118, https://doi.org/10.1007/s10841-007-9130-5. [ Links ]
Moir, M.L., Brennan, K.E.C., Majer, J.D., Fletcher, M.J. & Koch, J.M., 2005, 'Toward an optimal sampling protocol for Hemiptera on understorey plants', Journal of Insect Conservation, 9, 3-20, https://doi.org/10.1007/s10841-004-2351-y. [ Links ]
Muelelwa, M.I., Foord, S.H., Dippenaar-Schoeman A.S. & Stam, E.M., 2010, 'Towards a standardized and optimized protocol for rapid biodiversity assessments: spider species richness and assemblage composition in two savanna vegetation types', African Zoology, 45, 273-290, https://doi.org/10.3377/004.045.0206. [ Links ]
Munyai, T. C. & Foord, S.H., 2011, 'Ants on a mountain: spatial, environmental and habitat associations along an altitudinal transect in a centre of endemism', Journal of Insect Conservation, 16, 677-695. [ Links ]
Munyai, T.C. & Foord, S.H., 2015,'An inventory of epigeal ants of the western Soutpansberg Mountain Range, South Africa', Koedoe: African Protected Area Conservation and Science, 57, 1-12. [ Links ]
Nsengimana, V., Kaplin, B.A., Francis, F. & Nsabimana, D., 2017, 'A comparative study between sampling methods for soil litter arthropods in conserved tree plots and banana crop plantations in Rwanda', International Journal of Development and Sustainability, 6, 900-913, https://hdl.handle.net/2268/216095. [ Links ]
Ozanne, C.M.P, 2005, 'Insect sampling in forest ecosystems, in S.R. Leather (ed.), From insect sampling in forest ecosystems, pp. 58-77, Wiley-Blackwell, Oxford, UK, http://ndl.ethernet.edu.et/bitstream/123456789/39208/1/Simon%20R.%20Leather_2005.pdf. [ Links ]
Parr, C.L. & Chown, S.L., 2001, 'Inventory and bioindicator sampling: Testing pitfall and Winkler methods with ants in a South African savanna', Journal of Insect Conservation, 5, 27-36, https://doi.org/10.1023/A:1011311418962. [ Links ]
Patrick, M., Fowler, D., Dunn, R.R. & Sanders, N.J., 2012, 'Effects of treefall gap disturbances on ant assemblages in a Tropical Montane Cloud Forest', Biotropica, 44, 472-478, https://doi.org/10.1111/j.1744-7429.2012.00855.x. [ Links ]
Prasifka, J.R., Lopez, M.D., Hellmich, R.L., Lewis, L.C. & Dively, G.P., 2007, 'Comparison of pitfall traps and litter bags for sampling ground-dwelling arthropods', Journal of Applied Entomology, 131, 115-120, https://doi.org/10.1111/j.1439-0418.2006.01141.x. [ Links ]
Privet, K., Vedel, V., Fortune, C., Orivel, J., Martinez, Q., Cerdan, A., Baraloto, C. & Pétillon, J., 2020, 'Relative efficiency of pitfall trapping vs. nocturnal hand collecting in assessing soil-dwelling spider diversity along a structural gradient of Neotropical habitats', Diversity, 12, 1-11, https://doi.org/10.1080/01650521.2020.1806008. [ Links ]
Rejmánek, M. & Richardson, D.M., 2013, 'Trees and shrubs as invasive alien species - 2013 update of the global database', Diversity Distribution, 19, 1093-1094, https://on-linelibrary.wiley.com/doi/full/10.1111/ddi.12075. [ Links ]
Robertson, M.P, Harris, K.R., Coetzee, J.A., Foxcroft, L.C., Dippenaar-Schoeman, A.S. & Van Rensburg, B.J., 2011, 'Assessing local scale impacts of Opuntia stricta (Cactaceae) invasion on beetle and spider diversity in Kruger National Park, South Africa', African Zoology, 46(2), 205-223, https://doi.org/10.1080/15627020.2011.11407496. [ Links ]
R Core Team., 2017, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/. [ Links ]
Sheikh, A.H., Ganaie, G.A., Thomas, M., Bhandari, R. & Rather, Y.A., 2018, 'Ant pitfall trap sampling: An overview', Journal of Entomological Research, 42, 421-436, https://www.cabdirect.org/cabdirect/abstract/20183388873. [ Links ]
Siewers, J., Schirmel, J. & Buchholz, S., 2014, 'The efficiency of pitfall traps as a method of sampling epigeal arthropods in litter-rich forest habitats', European Journal of Entomology, 111, 69-74, https://www.eje.cz/pdfs/eje/2014/01/08.pdf. [ Links ]
Silva, F.H.O., Delabie, J.H.C., Dos Santos, G.B., Meurer, E. & Marques, M.I., 2013, 'Mini-Winkler extractor and pitfall trap as complementary methods to sample Formicida', Neotropical Entomology, 42, 351-358, https://doi.org/10.1007/s13744-013-0131-7. [ Links ]
S0rensen, L.L., Coddington, J.A. & Scharff, N., 2002, 'Inventorying and estimating sub-canopy spider diversity using semiquantitative sampling methods in an Afromontane forest', Environmental Entomology, 31, 319-330, https://doi.org/10.1603/0046-225X-31.2.319. [ Links ]
Thayer, M. & Werner, W., 2007, 'Terrestrial arthropod biodiversity: Planning a study and recommended sampling techniques', Biological Survey of Canada, 1, 1-32. [ Links ]
Tourinho, L.S., Dias, S.C., Lo Man Hung, N.F., Bonaldo, A.B., Pinto-da-Rocha, R. & Baccaro, F.B., 2018, 'Optimizing survey methods for spiders and harvestmen assemblages in an Amazonian upland forest', Pedobiologia, 67, 35-44, https://doi.org/10.1101/093740. [ Links ]
Tuf, I.H., 2015, 'Different collecting sampling methods reveal different ecological groups of centipedes (Chilopoda)', Journal of Zoologia 32, 345-350, http://dx.doi.org/10.1590/S1984-46702015000500003. [ Links ]
Zou, Y., Feng, J., Xue, D., Sang, W. & Axmacher, J.C., 2012, 'A comparison of terrestrial arthropod sampling methods', Journal of Resource Ecology, 3, 174-182, http://www.jorae.cn/EN/10.5814/j.issn.1674-764x.2012.02.010. [ Links ]
 Correspondence:
Correspondence:
Vanessa L. Lauchande
e-mail: vanessalauchande@gmail.com
Submitted: 6 April 2023
Accepted: 13 November 2023
Published: 19 April 2024














