Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.54 n.1 Pretoria 2024
http://dx.doi.org/10.38201/btha.abc.v54.1
ORIGINAL RESEARCH
The influence of distance from crushed stone mining on surface-active arthropods and soil chemical properties
Inam Yekwayo Asabonga Mngeni
Department of Biological and Environmental Sciences, Walter Sisulu University, Private Bag X1, Mthatha 5117, South Africa
ABSTRACT
BACKGROUND: Mining of crushed stone for use in construction is among the various anthropogenic activities in the Eastern Cape province in South Africa that alter natural landscapes. However, little is known about the impact of these activities on arthropods and soil chemical properties.
OBJECTIVES: We investigated the effect of distance (5, 30, 50 and 70 metres) from the mining sites on species richness, abundance and composition of surface-active arthropods, as well as composition and concentration of soil chemical properties (soil pH, calcium, magnesium, phosphorus and zinc.
METHODS: The study was conducted at two mining sites in Nyandeni Local Municipality within the O.R. Tambo District Municipality that were commissioned in 2014. Arthropods were sampled using pitfall traps and thereafter sorted into morphospecies, while an auger was used to collect soil samples. Permutational multi-variate analysis was used to compare the composition of the arthropods and soil chemical properties among distances from the mining activities, while arthropod species richness and abundance, as well as concentrations of soil chemical properties, were analysed using the one-way analysis of variance or Kruskal-Wallis test.
RESULTS: Arthropod species richness, abundance and composition were not influenced by distance from mining activities. Although among soil chemical properties, mining activities altered the concentration of zinc only, we found dissimilarities in soil composition between the sampling point at the edge of the mining activities and sampling points that were away from the mining sites.
CONCLUSION: We found no evidence of the impact of crushed stone mining on surface-active arthropods; however, our study revealed a negative impact of crushed stone mining on soil chemical properties.
Keywords: ants; beetles; spiders; species richness; Ikwezi mining site; Blue Rock mining site.
Introduction
Mining of stone is important because crushed stone is a construction material for building houses, roads and pavements (Adeyi et al. 2019). However, crushed stone mining has been reported to have a negative impact on the environment (Belay et al. 2020; Kalu & Ogbonna 2021; Otaraku et al. 2019; Pal & Mandal 2017). For example, pollution of water bodies by dust from crushed stone mining activities alters the water quality because of increased sedimentation from the suspended dust particles, high pH levels and water colour that looks grey (Pal & Mandal 2017). Reduced water quality affects aquatic biodiversity negatively, with polluted water leading to the mortality of invertebrates, which reduces the available fish food (Thanigaivel et al. 2023). In addition, heavy metals can pollute the water bodies and negatively impact fish (Thanigaivel et al. 2023).
Generally, the negative effects of mining activities on biodiversity are both direct and indirect. Land degradation and vegetation clearing, which lead to biodiversity loss, are two examples of the direct impact (Otaraku et al. 2019; Sonter et al. 2018). Indirect impact may result from the dust produced from crushing stone, affecting plant species that are sensitive to stone dust (Pal & Mandal 2017). Mining has been shown to negatively impact the density and species richness of local woody plant species, which were found to be significantly greater in unmined sites (Belay et al. 2020). The changes in vegetation cover, diversity and richness of plants directly impact animal species that are dependent on vegetation for food and shelter (Elandalew et al. 2018). For example, the abundance and species richness of some arthropods is positively associated with plant-species richness (Blaise et al. 2022; Ebeling et al. 2018). In addition, mining activities can lead to the establishment of invasive alien plant species, which reduce species richness of arthropods (Litt et al. 2014; Sonter et al. 2018).
Habitat fragmentation may lead to unfavourable living conditions for some animals and therefore trigger migration (Sonter et al. 2018). However, migration is only possible for animals with greater dispersal ability but limited in animals with poor dispersal abilities (Angelova et al. 2020). For example, ground-living arthropods occurring in natural forests did not overlap into the adjacent habitats due to their limited dispersal abilities (Perry et al. 2017; Yekwayo et al. 2017). In addition, mining activities alter habitat structure by decreasing the interior habitats, while increasing the edge. Fragmentation of habitats may influence arthropods negatively because edges decrease species richness and abundance of arthropods and also change assemblages unlike the interior habitats (With & Pavuk 2012).
Besides clearing vegetation, mining activities may contaminate the soil by changing the concentration of nutrients (Adewole & Adesina 2011; Wang et al. 2022). In Nigeria, soil samples in the stone mining area had greater concentrations of metals (nickel, chromium, cadmium, lead, copper, zinc and iron) compared to soil samples outside the mining area (Otaraku et al. 2019). Migliorini et al. (2004) recorded a high abundance of the Collembola, Diplura and Protura in soils with high concentrations of lead, while the Symphyla was not recorded in soils contaminated with lead. Therefore, arthropods respond differently to concentrations of metals in the soil. Furthermore, Lock et al. (2003) reported that an increase in zinc concentration in the soil led to a decrease in the abundance and species richness of springtails. Additionally, Reihart et al. (2021) also showed that greater concentration of nutrients may encourage the spread of invasive ant species, such as Nylanderia fulva, which was more abundant in calcium-contaminated soils than in soils without calcium. However, a combination of calcium and potassium was reported to decrease abundance of detritivorous arthropods (Reihart et al. 2021).
Alam et al. (1999) indicated that soil in mining areas tends to be acidic, while Anju and Banerjee (2011) reported that soil was either neutral or alkaline in metal mining areas. In stone mining areas, the soil pH was reported to be alkaline compared to areas where no mining took place (Belay et al. 2020). In addition, soil pH levels may influence the concentration of nutrients available in the soil (Alam et al. 1999; Zhao et al. 2011). For instance, Zhao et al. (2011) found that an increase in soil pH led to an increase in the concentration of calcium, magnesium, manganese and zinc, while soil pH correlated negatively with the concentration of phosphorus and nitrogen.
The concentration of nutrients available in the soil may be influenced by certain arthropod taxa, and that may affect plant growth (Almeida et al. 2019; Kaleri et al. 2020; Smit & Van Aarde 2001; Wagner et al. 1997). For example, activity of millipedes led to high concentration of calcium, phosphorus, potassium, carbon and nitrogen compared to soil that had no millipedes (Smit & Van Aarde 2001). However, different nutrients are influenced differently by the activity of ants (Almeida et al. 2019; Wagner et al. 1997). For instance, in nest soils, there were high concentrations of potassium, phosphorus, nitrate and ammonium, while the concentration of calcium and magnesium did not differ between nest soils and soils that are away from ant nests (Wagner et al. 1997). In addition, activity patterns of ants can lead to an increase in the abundance and species richness of plants, this was evident when comparisons were made between soils in close proximity to and those further away from ant nests (Almeida et al. 2019). Furthermore, high concentrations of soil nutrients, such as, calcium, magnesium, phosphorus and potassium were recorded in soils that had dung beetles compared to soils with no dung beetles (Hanafy & El-Sayed 2012; Kaleri et al. 2020).
Understanding how crushed stone mining alters the concentration of nutrients in the soil is important, as changes in soil nutrients may affect surface-active arthropods directly and indirectly. For example, abundance of Nylanderia fulva correlates positively with the concentration of soil calcium (Reihart et al. 2021), while Rosa et al. (2019) reported that different families of spiders correlate with different soil chemical properties. For example, spider families, such as the Gnaphosidae, Lycosidae and Salticidae correlated with calcium, magnesium and potassium, while families, such as the Corinnidae, Theridiidae and Zodariidae correlated with aluminium (Rosa et al. 2019). In addition, as a heavy metal, the concentration of zinc has been reported to be higher at the edges of the stone-mining sites than further away from the mining sites (Kalu & Ogbonna 2021; Ogbonna et al. 2011). Excessively high and/or low concentrations of zinc can affect plant growth negatively (Mousavi 2011; Mousavi et al. 2012). Zinc can indicate the availability of other nutrients, since its availability to plants can be affected by other nutrients, such as phosphorus and potassium (Jiang et al. 2018). For example, an increase in the concentration of phosphorus lead to a decrease in the concentration of available zinc (Mousavi et al. 2012). In addition to soil pH, the current study focused on the following soil chemical properties, zinc, phosphorus and magnesium, all of which are essential nutrients for plant growth (Meng et al. 2021; Mousavi 2011; Yan & Hou 2018; White & Broadley 2003). Response of arthropods to mining activities, such as limestone quarries in Czech Republic (Tropek et al. 2010) and gravel mining in Austria (Zulka 2013) has been investigated. However, according to our knowledge there are no records of the effect of crushed stone mining on arthropods in South Africa, in particular the Eastern Cape. Yet crushed stone mining is one of the growing anthropogenic activities that are taking place in the province (Department of Mineral Resources and Energy).
The first objective of this study was to determine if species richness, abundance and composition of surface-active arthropods change with distance from the crushed stone mining activities. We expected greater species richness and abundance at sampling points further away from the mining sites (where there is less disturbance from mining) than in close proximity to the mining site. Additionally, we hypothesised that species composition of arthropods would vary among the distances from the crushed stone mining activities. Our second objective was to determine if the composition and concentration of soil chemical properties (phosphorus, calcium, magnesium, zinc and pH) change with distance from the mining activities. We expected mining activities to increase the concentration of soil chemical properties at the edge of the mining site compared to away from the mining sites. Furthermore, we expected variation in the composition of soil chemical properties among sampling points. Lastly, we established if any of the soil properties correlated with arthropod species richness and abundance. We hypothesise that different soil properties will affect species richness and abundance of arthropods differently.
Materials and methods
Study area and design
The study was conducted in the Nyandeni Local Municipality within the O.R. Tambo District Municipality in Eastern Cape, South Africa (Figure 1). The area is characterised by warm (temperatures ranging from 16°C to 28°C) and wet (average monthly precipitation of 64.1 mm) summers, while winters are cool (temperatures ranging from 7°C to 20°C) and dry (Nkamisa et al. 2022). Grassland is one of the dominant biomes in the Eastern Cape. However, the grassland in our study was exposed to various anthropogenic activities, such as conversion into agricultural landscapes (croplands and pastures), mining sites, human settlements, and invasion by alien plant species, such as Lantana camara. We investigated two crushed stone mining sites, Ikwezi (-31.529467°, 28.952350°) and Blue Rock (-31.645033°, 28.900633°), which are approximately 13 km apart (Figure 1).
The mining in both sites started in 2014. The active mining at the Ikwezi mining site is approximately 14 ha, while it is approximately 16 ha at the Blue Rock mining site. Although the sizes of these mining sites suggest small scale mining, it is worth noting that the two sites are not the only crushed stone mining sites in the O.R. Tambo District Municipality, thus the importance of studying their effects on arthropods and soil chemical properties. Ikwezi and Blue Rock mining sites occur within a matrix of different anthropogenic activities, including pastures grazed by different livestock (Figure S1). The pastures were the immediate vegetation surrounding the mining sites and were fragmented by roads (footpaths, and gravel and tar roads). In addition to the different grass species in the pastures, there were other plant species, including, aloes, Berkheya sp., Heli-chrysum sp., and Lippia sp. Furthermore, these pastures were very rocky. Ikwezi mining site was approximately 1 km away from human settlements, unlike the Blue Rock mining site, which was approximately 2 km away from human settlements. However, grazing by livestock occurred in pastures surrounding both mining sites.
Collection and identification of surface-active arthropods
We established nine line transects, five at the Ikwezi and four at the Blue Rock mining sites. Each line transect started from the edge of the mining site (5 m from mining activities) into the surrounding vegetation (pasture). Each transect had four sampling points at 5, 30, 50 and 70 m from the mining activities, and each sampling point was about 1 × 3 m in size. Line transects within the same mining site were > 250 m apart to minimise pseudo-replication. The elevation in the transects ranged from 753 - 910 m above sea level. Two of these transects were on fairly flat ground, whereas seven were steeper as they got further from the mining sites.
Given that arthropods are known to be active mostly during the warmer months (Mavasa et al. 2022) and have been reported to correlate positively with precipitation (Uhey et al. 2020), we sampled in October 2021. Since the study was focused on surface-active arthropods, the pitfall trapping method was used. Pitfall traps were plastic cups that were 6 cm in diameter and 8 cm deep. According to Samways et al. (2010) traps collect arthropods based on the activity patterns, of which different species may be active at different times of the day and their activity may be influenced by weather patterns. As a result, we sampled arthropods over a period of 28 days. According to Jiménez-Carmona et al. (2019) digging-in effects can be accounted for by opening pitfall traps for > 48 hours, because digging-in effects occur within the first 24 hours after traps have been set out. Therefore, pitfall traps containing 50% of ethylene glycol solution were set out and left open for seven days. Samples were collected from traps on the 7th day, and new traps were set out in the same holes. The process of setting out pitfall traps was repeated four times within 28 days. At each sampling point (5, 30, 50 and 70 m) six pitfall traps that were approximately a metre apart were set out in the shape of a rectangle. Arthropod samples from the six pitfall traps, across all four sampling times at each sampling point, were pooled to form a single sample and preserved in one jar. Specimens were preserved in 70% ethanol before being sorted into morphospecies. Ants were sorted to subfamilies and genus where possible, spiders and beetles were sorted to family level, while cockroaches, millipedes, centipedes, scorpions and pseudoscorpions were identified to order level. Different identification guides were used when sorting specimens (Bouchard 2014; Dippenaar-Schoeman 2014; Dippenaar-Schoeman & Jocqué 1997; Fisher & Bolton 2016; Holm & Dippenaar-Schoeman 2010; Picker et al. 2019; White 1998). The use of morphospecies may lead to over-splitting and lumping of species, however, we used them because Derraik et al. (2002) showed that these limitations only become pronounced when there is a lack of experience with sorting specimens. In our study, the first author, whom has several years of experience, sorted specimens into morphospecies. Voucher specimens are currently housed at the Department of Biological and Environmental Sciences, Walter Sisulu University, however, these voucher specimens will later be housed in the KwaZulu-Natal Museum, Pietermaritzburg.
Collection of soil samples
A total of five soil samples at each sampling point were collected using an auger (8.5 cm in diameter and 14.5 cm deep). The five soil samples were mixed in a bucket to form a sample for each sampling point. We collected soil samples once within the 28 days; however, we acknowledge that it is possible that rainfall, which occurred in some days during this period, may have interfered with available nutrients in the soil surface. Soil samples were stored in Ziploc bags before being sent to the soil analytical laboratory at the Department of Agriculture, Land Reform and Rural Development. The soil chemical properties that were analysed included pH and concentration of phosphorus, calcium, magnesium and zinc.
Data analyses
Although arthropods were collected over four sampling periods, we did not account for repeated sampling in the analyses because samples were pooled immediately after collection from the field and treated as a single sample. Given that the two mining sites were about 13 km apart, we first explored our taxonomic data by testing whether there were differences in species composition of arthropods between the two mining sites. Permutational multivariate analysis of variance (PERMANOVA) in PRIMER 7 was used to compare arthropod assemblages between the mining sites (Anderson 2017). Permutational multivariate analysis of variance showed that the two mining sites support significantly different assemblages of arthropods (PseudoF = 4.78, df = 1, p = 0.0001), indicating that there may be other factors that are unique to each site that are affecting arthropods. As a result, we could not treat transects from the two mining sites as replicates. Instead, we analysed data from each mining site separately. There were five and four line transects at Ikwezi and Blue Rock mining sites respectively.
Given that the pitfall trapping method was used to sample arthropods, we studied incidence data rather than the total abundance data of the sites. As such, the non-parametric estimators (Chao2 and Jackknife2) that are based on incidence data were used to determine whether sampling was sufficient (Chao & Chiu 2016; Hortal et al. 2006). Species richness was estimated in PRIMER 7.
The effect of distance from the mining sites on the composition of arthropods and soil nutrients was determined using PERMANOVA in PRIMER 7. Arthropod data were square-root transformed to reduce the weight of common species, and Bray-Curtis similarity measures with 9999 permutations were used (Anderson et al. 2008). Soil nutrient data were log transformed, normalised and analyses were performed using the Euclidean distance with 9999 permutations (Anderson et al. 2008). Principal coordinates analysis in PRIMER were used to visualise the effect of distance from the mining sites on the composition of soil chemical properties (Anderson et al. 2008).
Species richness and abundance of arthropods, as well as concentrations of soil chemical properties were tested for normality using the Shapiro-Wilk test and the Quantile-Quantile plots in R version 4.1.2 (R Development Core Team 2021). The output of the Shapiro-Wilk test can be affected by the sample size; thus we also used the Quantile-Quantile plots with ggpubr package (Kassambara & Kassambara 2020; Rochon et al. 2012). Species richness of arthropods at both mining sites were normally distributed, while the arthropod abundance at both sites were not normally distributed. At Blue Rock mining site, datasets for magnesium and zinc were normally distributed, while datasets for calcium, phosphorus and pH were not normally distributed. At Ikwezi mining site, datasets for soil chemical properties (except for phosphorus) were normally distributed. One-way analysis of variance (ANOVA) compares the means among more than two samples/observations that are normally distributed (Bewick et al. 2004). Therefore, for arthropod and soil nutrient datasets that were normally distributed we used the one-way ANOVA. However, for datasets of arthropods and soil chemical properties that were not normally distributed, we used the non-parametric Kruskal-Wallis test. The Kruskal-Wallis test is a useful statistical tool when examining data that defy normality assumptions, and it is used to compare means of three or more independent groups (Lee 2022). The Tukey multiple comparison post hoc test was used to show differences between paired sampling points (5, 30, 50 and 70 m). Analysis of variance and the Kruskal-Wallis test were performed in R version 4.1.2 (R Development Core Team 2021). Spearman's rank correlations in R version 4.1.2 (R Development Core Team 2021) were used to determine if soil chemical properties correlated with arthropod species richness and abundance.
Results
Number of individuals and morphospecies sampled at Blue Rock mining site
A total of 7 121 individuals in eight arthropod taxa (ants, beetles, spiders, cockroaches, millipedes, centipedes, scorpions and pseudoscorpions) were collected (Table S1). There were 277 morphospecies, of which 51% were singletons and doubletons. There were differences between the observed and estimated species richness (observed = 277, Chao2 = 385.21±26.86, Jacknife2 = 433.63). Out of the three dominant taxa, ants were the most abundant with 5 600 individuals from 46 morphospecies. Collected ants belonged to four subfamilies, with the Myrmicinae and Formicinae being most abundant and species rich, while few individuals and morphospecies were in the Dorylinae, Ponerinae and Dolichoderinae (Table S1). Spiders were represented by 861 individuals and 109 morphospecies. The highest abundance of spiders was in the Ly-cosidae, Gnaphosidae, Salticidae and Zodariidae (Table S1), whereas the Ammoxenidae, Pholcidae, Pisauridae and Selenopidae were represented by a single individual each (Table S1). The most species-rich families of spiders were the Lycosidae and Salticidae, while many families had a single morphospecies each (Table S1).
Beetles were the least abundant group within the three dominant taxa, with 449 individuals in 91 morphospecies. The most abundant families of beetles were the Staphylinidae, Curculionidae, Scarabaeidae, Tenebrionidae and Carabidae (Table S1). The Histeridae and Niti-dulidae had two individuals each, while the Hydrophilidae and Cleridae were represented by a single individual each (Table S1). Beetle families with high numbers of morphospecies were the Staphylinidae, Curculionidae, Scarabaeidae, Chrysomelidae and Carabidae, while the least species-rich families were Hydrophilidae and Cleridae with one morphospecies each (Table S1).
Number of individuals and morphospecies sampled at Ikwezi mining site
There were 9 808 individuals in 292 morphospecies (47% were singletons and doubletons) of ants, beetles, spiders, cockroaches and centipedes collected (Table S1). There were differences between the observed and the estimated species richness (observed = 292, Chao2 = 424.25±33.02, Jacknife2 = 462.12). Out of the three dominant taxa, ants had 5 596 individuals in 40 morphospecies, spiders had 2 132 individuals in 110 morphospecies, while beetles had 1 950 individuals in 109 morphospecies. Ants followed the same patterns as the one at the Blue Rock site, with the Myrmicinae and Formicinae being greater contributors in abundance and species richness, while the opposite was true for the Dorylinae, Ponerinae and Dolichoderinae (Table S1). When it comes to spiders, the Lycosidae, Gnaphosidae and Zodariidae were the most abundant families (Table S1). The highest number of morphospecies were in the Lycosidae and Salticidae, while many families had a single morphospecies (Table S1). The Staphylinidae, Scarabaeidae, Elateridae, Carabidae, Curculionidae, Chrysomelidae and Scydmaenidae had the highest number of individuals of beetles (Table S1). The most species-rich families of beetles were the Staphylinidae, Scarabaeidae, Curculionidae and Carabidae, while other families were represented by six, three, two or one morphospecies (Table S1).
Effect of distance from mining sites on surface-active arthropods and soil chemical properties
Species composition of arthropods was not significantly influenced by distance from the mining sites (both Blue Rock and Ikwezi) (Table 1). Species richness (df = 3, SS = 112.2, MS = 37.40, F = 0.43, p = 0.74) and abundance (χ2 = 2.63, df = 3, p = 0.45) at Blue Rock did not differ among the sampling points from the mining site. Similarly, distance from Ikwezi mining site did not affect species richness (df = 3, SS = 23, MS = 7.65, F = 0.08, p = 0.97) and abundance (χ2 = 1.81, df = 3, p = 0.61).
Permutational multivariate analysis of variance showed that the composition of soil chemical properties is significantly influenced by the distance from the mining sites (Table 1). At Blue Rock mining site, the first axis contributed 44.9% of the total variation in composition of soil chemical properties when compared to the second axis, which contributed 25.3% (Figure 2A). The first and second axes contributed 38.2% and 30.8%, respectively, towards variations in the composition of soil chemical properties among distances from the Ikwezi mining site (Figure 2B). At Blue Rock mining site, significant differences in soil nutrient composition were detected between the 5 and 50 m sampling points, 5 and 70 m sampling points, as well as between 30 and 70 m sampling points (Table 1, Figure 2A). Sampling points that were in close proximity to each other (5 and 30 m, 30 and 50 m, as well as 50 and 70 m) at Blue Rock mining site did not differ in soil nutrient composition (Table 1, Figure 2A). However, at Ikwezi mining site the soil nutrient composition at the 70 m sampling point was significantly different from the composition at 5 and 30 m sampling points (Table 1, Figure 2B). In contrast, no other pairs showed differences at Ikwezi mining site (Table 1, Figure 2B).
Distance from the mining sites (Blue Rock and Ikwezi) significantly affected the zinc concentration, while other soil chemical properties were not significantly affected (Table 2). The concentration of zinc at Blue Rock mining site differed between 5 and 30 m (p = 0.02), 5 and 50 m (p < 0.001), 5 and 70 m (p < 0.001), 30 and 50 m (p = 0.03), as well as 30 and 70 m (p = 0.001) (Figure 3A). However, the concentration of zinc at Blue Rock mining site did not differ (p = 0.29) between 50 and 70 m sampling points (Figure 3A). At Ikwezi mining site the pairwise comparisons showed significant differences in the concentration of zinc between all paired sampling points, 5 and 30 m (p = 0.002), 5 and 50 m (p < 0.001), 5 and 70 m (p < 0.001), 30 and 50 m (p = 0.003), 30 and 70m (p < 0.001), 50 and 70 m (p = 0.01) (Figure 3B). In both mining sites, greater concentrations of zinc were recorded at 5 m from the mining sites, while the lowest were at 70 m from the mining sites (Figure 3).
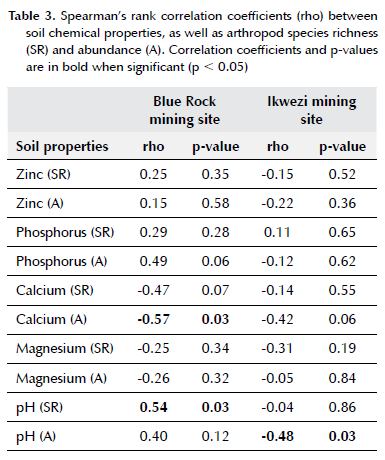
Species richness and abundance of arthropods did not correlate with most soil chemical properties (except for calcium and pH) (Table 3). At Blue Rock mining site, a negative correlation (rho = -0.57, p = 0.03) between calcium and arthropod abundance was recorded (Table 3). A positive correlation (rho = 0.54, p = 0.03) between arthropod species richness and soil pH was recorded at Blue Rock mining site (Table 3). Arthropod abundance was negatively correlated with soil pH (rho =-0.48, p = 0.03) at Ikwezi mining site (Table 3).
Discussion
Effect of distance from the crushed stone mining sites on surface-active arthropods
In this study, we expected species richness and abundance of surface-active arthropods to increase with increasing distance from the mining activities, and species composition to change with distance from the mining activities. However, the distance from the mining activities did not affect species richness, abundance or composition. This was surprising considering the fact that crushed stone mining is an anthropogenic activity, of which some anthropogenic activities affect some arthropods negatively. For example, when compared to conserved sites Rivas-Arancibia et al. (2014) reported a decrease in abundance of Pheidole skwarrae, P. tepicana, Pogonomyrmex barbatus and Dorymyrmex pyramicus in sites disturbed by livestock grazing, human activities and land degradation. However, abundance of Brachymymex musculus increased in disturbed sites compared to undisturbed sites (Rivas-Arancibia et al. 2014). Similarities in species richness, abundance, and composition of surface-active arthropods among the different distances from the mining sites may be because the mining sites that we sampled are like 'islands' that occur within a matrix of other anthropogenic activities. This matrix included pastures that are fragmented by roads and residential areas.
Savage et al. (2018) used the disturbance tolerance hypothesis and reported that arthropods, which occur in disturbed habitats, tend not to be affected by drastic or severe disturbance compared to those occurring in less disturbed habitats. As such, arthropods in our study are probably not affected by crushed stone mining activities because they exist in disturbed areas, and they may have become tolerant of the disturbance. Furthermore, in Denmark, disturbance-adapted species of arthropods led to greater species richness in disturbed habitats compared to undisturbed habitats (Brunbjerg et al. 2015). High arthropod diversity in disturbed habitats may be due to species that are early colonisers of disturbed sites and species that can tolerate disturbance (McCluney & Sabo 2014). McCluney and Sabo (2014) also found greater diversity of carabids (early colonisers) near artificial pools, and greater diversity of wolf spiders in sites that were temporarily dry, indicating their tolerance to habitat changes. The resilience of spiders to habitat change was also evident when Lowe et al. (2017) recorded similarities in abundance among three habitat types (urban parks, remnant vegetation and bushland sites). Therefore, similarities in species richness, abundance and composition of spiders among the four sampling points in our study may be linked to resilience of spiders to habitat change.
Of the arthropods that we collected, the abundant and speciose taxa were ants, spiders and beetles. Given that spiders, some ants and some beetles are predators, they occupy a variety of habitats because they are influenced by the availability of prey. Some ant species are generalist foragers that occupy different habitats (Rivas-Arancibia et al. 2014), this may explain the similarities in species richness, abundance and composition among our sampling points. In addition, two of the most abundant and species-rich families of beetles (ground and rove beetles) are predators, which can occupy diverse habitats. Microhabitats for rove beetles include leaf litter, dung, decaying matter and underneath stones (Picker et al. 2019). In our study, dung from grazing livestock was readily available in the pasture areas. The availability of dung in the pastures that serves as food for dung beetles may have led to their high species richness and abundance in our sampling points (Picker et al. 2019).
The surrounding matrix can potentially influence the density and composition of arthropods in a habitat occurring within that matrix; for example, arthropod density in a grassland surrounded by crop fields (Madeira et al. 2016), and arthropod composition in natural forests surrounded by pine plantations or grassland (Yekwayo et al. 2016). In our study, the influence of the surrounding matrix can be used to explain our results because the crushed stone mining sites are a disturbance occurring in a matrix of pastures. As such, microhabitats within the pastures may have led to high arthropod richness and abundance in our sampling points.
Similarities in species composition of arthropods across our sampling points may also have been due to the short distance among points and the uniformity of vegetation type around the mining sites. Swart et al. (2018) found that assemblages of arthropods in the interior of the natural forest (100 m from the edge) overlapped into the adjacent habitats. However, the extent of overlap was dependant on the type of the adjacent habitat and arthropod taxa (Swart et al. 2018). For example, natural forest assemblages of arachnids overlapped up to 10 m from the edge into the plantation blocks that had been cleared for rehabilitation, while significant differences were observed at 50 m into pine plantations (Swart et al. 2018). These results agree with those of Yekwayo et al. (2017), which reported that contrasting habitats (natural forest and grassland) showed no overlap of arthropod assemblages, while overlaps were detected between structurally similar habitats (natural forest and pine). As such, similarities in assemblages across sampling points in our study may be linked to the fact that the mining sites were surrounded by grassland (pasture). On the other hand, Swart et al. (2018) showed that beetles are more sensitive to habitat changes than the other taxa with natural forest assemblages not overlapping into the adjacent habitats, while differences in ant assemblages were observed at 20 m into pine and plantation blocks cleared for rehabilitation. Therefore, the argument of shorter distances among our sampling points may not have been the case for all the taxa, given that most of these taxa have limited dispersal abilities.
Effect of distance from the crushed stone mining sites on soil chemical properties
Given that crushed stone mining activities increase the amount of dust produced (Opondo et al. 2022; Pal & Mandal 2017), it is possible that this dust interfered with the soil nutrient composition. The impact of dust on soil chemical properties might have been more severe in close proximity to the mining sites than further away. Thus, we found differences in the composition of soil chemical properties between the sampling point at the edge of the mining sites compared to those further away from the mining activities (50 and 70 m). Although dolerite stone was mined in our sites, Tonello et al. (2021) found that dust from mining agate stone led to changes in soil chemical properties. According to Alloway (2008) weathering of rocks is one of the factors that determine the concentration of zinc in the soil. Therefore, it was not surprising that we recorded greater concentration of zinc at sampling points that are closer to the crushed stone mining sites than those further away. Our results agree with those of a study by Otaraku et al. (2019) in Nigeria, which recorded greater concentration of metals (including zinc) near the rock-crushing area compared to further away. We show that the concentration of zinc in the soil can be used as an indicator of disturbance by crushed stone mining, since the concentrations of other soil chemical properties (calcium, magnesium, phosphorus and pH) did not differ among sampling points. Pal and Mandal (2021) showed that wind can facilitate the spread of stone dust into the surrounding areas, which may increase the size of the areas affected. Therefore, similarities in concentrations of calcium, magnesium, phosphorus and pH among our sampling points may have resulted from crushed stone pollution that affected soils adjacent to the mining sites. Pollution may have been facilitated because the gradients of seven out of the nine transects were steeper with the distance from the mining sites. Our results support previous studies that reported that different soil chemical properties are influenced by crushed stone mining activities differently. For example, in India, Pal and Mandal (2021) recorded alkaline soil pH, low concentration of nitrogen, phosphorous, copper and manganese, while there was higher concentration of zinc near crushed stone activities compared to further away from mining sites. Furthermore, in Nigeria, Adewole and Adesina (2011) found reduced concentration of phosphorus near the mining sites compared to further away from the marble-mining sites.
Surface-active arthropods and soil chemical properties
According to Ashford et al. (2013) the concentration of soil chemical properties (calcium, magnesium, nitrate, carbon, nitrogen and pH) can be influenced by leaf litter depth, with greater concentrations of these properties in plots with deeper leaf litter. Furthermore, abundance of Acari, Araneae, Coleoptera, Diplura and Formicidae increase with an increase in leaf litter depth (Ashford et al. 2013; Silveira et al. 2010). However, in our study, positive correlation was recorded between soil pH and species richness at Blue Rock mining site. In contrast, no other positive correlation was observed between the measured soil properties, and arthropod species richness or abundance. Compared to the study by Ashford et al. (2013), there was no deeper layer of leaf litter in our sites, but the leaf litter was sparsely distributed in our sampling points, and this was not surprising, considering that grass was the dominant vegetation type. Furthermore, Melliger et al. (2018) found a negative correlation between species richness of ants and litter pH. In our study a negative correlation was recorded between arthropod abundance and soil pH at Ikwezi mining site. Although at Blue Rock mining site, a significant moderate positive correlation was recorded between soil pH and species richness, we also observed a non-significant positive correlation between soil pH and arthropod abundance. These results support Zhao et al. (2017) who found a weak positive correlation between arthropod abundance and soil pH.
Sayer et al. (2010) reported that phosphorus, calcium and sodium concentrations are vital in shaping the diversity of arthropods, while the concentrations of sodium and magnesium are vital for arthropod abundance. Even though the soil concentration of calcium in our study did not differ among our sampling points, at Blue Rock mining site, there was a significant strong negative correlation between calcium and arthropod abundance and a non-significant moderate negative correlation between calcium and arthropod species richness. Furthermore, Van der Wal et al. (2009) found a positive correlation between the soil concentration of calcium and the diversity and evenness of plant species, of which herbivorous arthropods increase with plant diversity (Barnes et al. 2020). Therefore, we did not expect a negative correlation between soil concentration of calcium and arthropod species richness and abundance, especially because of the high contents of calcium that Graveland and Van Gijzen (1994) recorded in different arthropod taxa. However, crushed stone mining activities in our study may have increased calcium concentration beyond what arthropods can tolerate. In our study, the zinc concentration in the soil varied across sampling points, with more concentration at 5 m than at 70 m from the mining sites. However, arthropod species richness and abundance did not differ among sampling points. Variations in correlations between soil properties and arthropod species richness and abundance from the two mining sites indicate that other factors (other than crushed stone mining) influence arthropods and soil properties.
Conclusion
Our results found no evidence that crushed stone-mining activities affected assemblages of surface-active arthropods in adjacent vegetation. Crushed stone mining activities did, however, seem to result in an increased concentration of zinc. Although concentrations of other soil chemical properties (calcium, magnesium, phosphorus and pH) did not vary among distances from the mining activities, we found that composition of soil nutrients varied among distances, with greater dissimilarities between the edge (5 m from the mining sites) and sampling points further away from the mining sites.
Acknowledgements
The authors gratefully acknowledge the Blue Rock and Ikwezi mining sites for permission to conduct the study. We are thankful to Mr Sibonelo Mhlongo who assisted with fieldwork, as well as Professor Tarombera Mwabvu for arthropod (millipedes and beetles) identification. We thank the soil analytical laboratory (Department of Agriculture, Land Reform and Rural Development) at Mthatha Dam. In addition, we thank the editors and two anonymous reviewers for their contributions in improving our manuscript.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
I.Y. did the conceptualisation, investigation, methodology, data curation, formal analyses and wrote the original draft. A.M. did the conceptualisation, investigation, methodology, and reviewed and edited the article.
References
Adewole, M.B. & Adesina, M.A., 2011, 'Impact of marble mining on soil particles in a part of Guinea savanna zone of southwestern Nigeria', Ethiopian Journal of Environmental Studies and Management, 4(2), 1-8, https://doi.org/10.4314/ejesm.v4i2.1. [ Links ]
Alam, S.M., Naqvi, S.S.M. & Ansari, R., 1999, 'Impact of soil pH on nutrient uptake by crop plants', in M. Pessarakli (ed.), Handbook of Plant and Crop Stress, Marcel Dekker Inc., New York. [ Links ]
Adeyi, G.O., Mbagwu, C.C., Ndupu, C.N. & Okeke, O.C., 2019, 'Production and uses of crushed rock aggregates: an overview', International Journal of Advanced Academic Research, 5(8), 92-110. [ Links ]
Alloway, B.J., 2008, Zinc in Soils and Crop Nutrition, International Zinc Association and International Fertilizer Industry Association, Paris. [ Links ]
Almeida, F.S., Elizalde, L., Silva, L.M.S. & Queiroz, J.M., 2019, 'The effects of two abundant ant species on soil nutrients and seedling recruitment in Brazilian Atlantic Forest', Revista Brasileira de Entomologia, 63(4), 296-301, https://doi.org/10.1016/j.rbe.2019.08.001. [ Links ]
Anderson, M.J., 2017, 'Permutational multivariate analysis of variance (PERMANOVA)', Wiley Stats Ref: Statistics Reference Online, https://doi.org/10.1002/9781118445112.stat07841. [ Links ]
Anderson, M.J., Gorley, R.N. & Clarke, K.R., 2008. PER-MANOVA+ for PRIMER: Guide to Software and Statistical Methods, PRIMER-E: Plymouth, United Kingdom. [ Links ]
Angelova, R.A., Velichkova, R. & Alexandrova, S., 2020, 'Environmental impact of quarry activities: the case study of a gneiss deposit in the region of Blagoevgrad', Proceeding of 1st International Conference on Environmental Protection and Disaster RISKs, 29-30 September 2020, Sofia, Bulgaria. [ Links ]
Anju, M. & Banerjee, D.K., 2011, 'Associations of cadmium, zinc, and lead in soils from a lead and zinc mining area as studied by single and sequential extractions', Environmental Monitoring and Assessment, 176, 67-85, https://doi.org/10.1007/s10661-010-1567-4. [ Links ]
Ashford, O.S., Foster, W.A., Turner, B.L., Sayer, E.J., Sutcliffe, L. & Tanner, E.V.J., 2013, 'Litter manipulation and the soil arthropod community in a lowland tropical rainforest', Soil Biology and Biochemistry, 62, 5 - 12, https://doi.org/10.1016/j.soilbio.2013.03.001. [ Links ]
Barnes, A.D., Scherber, C., Brose, U., Borer, E.T., Ebeling, A., Gauzens, B., Giling, D.P., Hines, J., Isbell, F., Ristok, C., Tilman, D., Weisser, W.W. & Eisenhauer, N., 2020, 'Biodiversity enhances the multitrophic control of arthropod herbivory', Science Advances, 6(45), https://doi.org/10.1126/sciadv.abb6603. [ Links ]
Belay, L., Birhane, E., Zenebe, A., Weldu, A., Chiemela, S.N. & Solomon, N., 2020, 'Effects of stone mining on woody plant species diversity and selected soil properties in northern Ethiopia', Environmental Systems Research, 9, 1-12, https://doi.org/10.1186/s40068-020-00171-8. [ Links ]
Bewick, V., Cheek, L. & Ball, J., 2004, 'Statistics review 9: one-way analysis of variance', Critical Care, 8, 130-136, https://doi.org/10.1186/cc2836. [ Links ]
Blaise, C., Mazzia, C., Bischof, A., Millon, A., Ponel, P. & Blight, O., 2022, 'Vegetation increases abundances of ground and canopy arthropods in Mediterranean vineyards', Scientific Reports, 12, 3680, https://doi.org/10.1038/s41598-022-07529-1. [ Links ]
Bouchard, P., 2014, The book of beetles: a life-size guide to six hundred of nature's gems, University of Chicago Press, Chicago. [ Links ]
Brunbjerg, A.K., Jørgensen, G.P, Nielsen, K.M., Pedersen, M.L., Svenning, J.C. & Ejrnæs, R., 2015, 'Disturbance in dry coastal dunes in Denmark promotes diversity of plants and arthropods', Biological Conservation, 182, 243-253, https://doi.org/10.1016/j.biocon.2014.12.013. [ Links ]
Chao, A. & Chiu, C.H., 2016, 'Species richness: estimation and comparison', Wiley Stats Ref: Statistics Reference Online, 1-26, https://doi.org/10.1002/9781118445112.stat03432.pub2. [ Links ]
Department of Mineral Resources and Energy, viewed 26 July 2023, from https://www.dmr.gov.za/mineral-policy-promotion/operating-mines/eastern-cape. [ Links ]
Derraik, J.G.B., Closs, G.P., Dickinson, K.J.M., Sirvid, P., Barratt, B.I.P. & Patrick, B.H., 2002, 'Arthropod morphospecies versus taxonomic species: a case study with Araneae, Coleoptera, and Lepidoptera', Conservation Biology, 16(4), 1015-1023, https://doi.org/10.1046/j.1523-1739.2002.00358.x. [ Links ]
Dippenaar-Schoeman, A.S., 2014, Field guide to the spiders of South Africa, Lapa Publishers, Cape Town. [ Links ]
Dippenaar-Shoeman, A.S. & Jocqué, R., 1997, African spiders: an identification manual, ARC-Plant Protection Research Institute, Pretoria. [ Links ]
Ebeling, A., Hines, J., Hertzog, L.R., Lange, M., Meyer, S.T., Simons, N.K. & Weisser, W.W., 2018, 'Plant diversity effects on arthropods and arthropod-dependent ecosystem functions in a biodiversity experiment', Basic and Applied Ecology, 26, 50-63, https://doi.org/10.1016/j.baae.2017.09.014. [ Links ]
Elandalew, A., Tasew, E. & Tolahun, S., 2018, 'Environment and social impacts of stone quarrying: South Western Ethiopia, in case of Bahir Dar Zuria Wereda Zezelma Kebele' International Journal of Research in Environmental Science, 5(2), 29 - 38, https://doi.org/10.20431/2454-9444.0502005. [ Links ]
Fisher, B.L. & Bolton, B., 2016, Ants of Africa and Madagascar: A guide to genera, University of California Press, California. [ Links ]
Graveland, J. & Van Gijzen, T., 1994, 'Arthropods and seeds are not sufficient as calcium sources for shell formation and skeletal growth in Passerines', Ardea, 55(1-2), 299-314, https://doi.org/10.5253/arde.v82.p299. [ Links ]
Hanafy, E.M.H. & El-Sayed, W., 2012, 'Soil nutrient as affected by activity of dung beetles, Scarabaeus sacer (Coleoptera: Scarabaeidae) and toxicity of certain herbicides on beetles', Journal of Applied Sciences Research, 8(8), 4752-4758. [ Links ]
Holm, E. & Dippenaar-Schoeman, A., 2010, Gogo guide: the arthropods of southern Africa, Lapa Publishers, Pretoria. [ Links ]
Hortal, J., Borges, P.A.V. & Gaspar, C., 2006, 'Evaluating the performance of species richness estimators: sensitivity to sample grain size', Journal of Animal Ecology, 75, 274-287. [ Links ]
Jiang, Y., Gao, W.W., Zhao, J.L., Chen, Q., Liang, D, Xu, C., Huang, L.S. & Ruan, L.M., 2018, 'Analysis of influencing factors on soil Zn content using generalized additive model', Scientific Reports, 8(1), 15567, https://doi.org/10.1038/s41598-018-33745-9. [ Links ]
Jiménez-Carmona, F., Carpintero, S. & Reyes-López, J.L., 2019. 'The digging-in effect on ant studies with pitfall traps: influence of type of habitat and sampling time' Entomologia Experimentalis et Applicata, 167, 906-914, https://doi.org/10.1111/eea.12834. [ Links ]
Kaleri, A.R., Ma, J., Jakhar, A.M., Hakeem, A., Ahmed, A., Napar, W.P.F., Ahmed, S., Han, Y., Abro, S.A., Nabi, F., Tan, C. & Kaleri, A.H., 2020, 'Effects of dung beetle-amended soil on growth, physiology, and metabolite contents of bok choy and improvement in soil conditions', Journal of Soil Science and Plant Nutrition, 20, 2671-2683, https://doi.org/10.1007/s42729-020-00333-8. [ Links ]
Kalu, I.E. & Ogbonna, N.J., 2021, 'Investigation of environmental effect of stone quarrying activities on soil and water in Akpoha and Ishiagu communities of Ebonyi state, Nigeria', International Journal of Construction Management, 21(12), 1185 - 1199, https://doi.org/10.1080/15623599.2019.1604115. [ Links ]
Kassambara, A. & Kassambara, M.A., 2020, 'Package gg-pubr', R package version 0.1.6. [ Links ]
Lee, S.W., 2022, 'Methods for testing statistical differences between groups in medical research: statistical standard and guideline of life cycle committee', Life Cycle, 2, e1, https://doi.org/10.54724/lc.2022.e1. [ Links ]
Litt, A.R., Cord, E.E., Fulbright, T.E. & Schuster, G.L., 2014, 'Effects of invasive plants on arthropods', Conservation Biology, 28(6), 1532-1549, https://doi.org/10.1111/cobi.12350. [ Links ]
Lock, K., Janssens, F. & Janssen, C.R., 2003, 'Effects of metal contamination on the activity and diversity of springtails in an ancient Pb-Zn mining area at Plombières, Belgium', European Journal of Soil Biology, 39(1), 25-29, https://doi.org/10.1016/S1164-5563(02)00006-7. [ Links ]
Lowe, E.C., Wilder, S.M. & Hochuli, D.F., 2017, 'Life history of an urban-tolerant spider shows resilience to anthropogenic habitat disturbance', Journal of Urban Ecology, 3(1), 1-10, https://doi.org/10.1093/jue/jux004. [ Links ]
Madeira, F., Tscharntke, T., Elek, Z., Kormann, U.G., Pons, X., Rösch, V., Samu, F., Scherber, C. & Batáry, P, 2016, 'Spillover of arthropods from cropland to protected calcareous grassland - the neighbouring habitat matters', Agriculture, Ecosystems and Environment, 235, 127-133, https://doi.org/10.1016/j.agee.2016.10.012. [ Links ]
Mavasa, R., Yekwayo, I., Mwabvu, T. & Tsvuura, Z., 2022, 'Preliminary patterns of seasonal changes in species composition of surface-active arthropods in a South African savannah', Austral Ecology, 47(6), 1222 - 1231, https://doi.org/10.1111/aec.13213. [ Links ]
McCluney, K.E. & Sabo, J.L., 2014, 'Sensitivity and tolerance of riparian arthropod communities to altered water resources along a drying river', PLoS One, 9, https://doi.org/10.1371/journal.pone.0109276. [ Links ]
Melliger, R.L., Braschler, B., Rusterholz, H.P & Baur, B., 2018, 'Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders', PLoS One, 13(6), https://doi.org/10.1371/journal.pone.0199245. [ Links ]
Meng, X., Chen, W.W., Wang, Y.Y., Huang, Z.R., Ye, X., Chen, L.S. & Yang, L.T., 2021, 'Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis', PloS One, 16(2), https://doi.org/10.1371/journal.pone.0246944. [ Links ]
Migliorini, M., Pigino, G., Bianchi, N., Bernini, F. & Leonzio, C, 2004, 'The effects of heavy metal contamination on the soil arthropod community of a shooting range', Environmental Pollution, 129, 331-340, http://doi.org/10.1016/j.envpol.2003.09.025. [ Links ]
Mousavi, S.R., 2011, 'Zinc in crop production and interaction with phosphorus', Australian Journal of Basic and Applied Sciences, 5(9), 1503-1509. [ Links ]
Mousavi, S.R., Galavi, M. & Rezaei, M., 2012, 'The interaction of zinc with other elements in plants: a review', International Journal of Agriculture and Crop Sciences, 4(24), 1881-1884. [ Links ]
Nkamisa, M. Ndleve, S., Nakin, V.M., Mngeni, A. & Kabiti, H.M., 2022, 'Analysis of trends, recurrences, severity and frequency of droughts using standardised precipitation index: Case of OR Tambo District Municipality, Eastern Cape, South Africa', Jàmbá: Journal of Disaster Risk Studies 14(1), http://dx.doi.org/10.4102/jamba.v14i1.1147. [ Links ]
Ogbonna, PC., Emea, R. & Da Silva, J.A.T., 2011, 'Heavy metal concentration in soil and woody plants in a quarry', Toxicological and Environmental Chemistry, 93(5), 895-903, https://doi.org/10.1080/02772248.2011.564361. [ Links ]
Opondo, E.O., Ajayi, D.D. & Makindi, S.M., 2022, 'Impacts of quarrying activities on the environment and livelihood of people in Border II sub-location, Nyando sub-county, Kisumu County, Kenya', Environmental Quality Management, 32(3), 147 - 160, https://doi.org/10.1002/tqem.21881. [ Links ]
Otaraku, I.J. Nwambo, Y.P & Egun, I.L., 2019, 'The impact of rock crushing on the quality of air and soil within and outside crushing site', British Journal of Environmental Sciences, 7(1), 1-7. [ Links ]
Pal, S. & Mandal, I., 2017, 'Impacts of stone mining and crushing on stream characters and vegetation health of Dwarka river basin of Jharkhand and West Bengal, Eastern India', Journal of Environmental Geography, 10(1-2), 11-21. [ Links ]
Pal, S. & Mandal, I., 2021, 'Impacts of stone mining and crushing on environmental health in Dwarka river basin', Geocarto International, 36(4), 392-420, https://doi.org/10.1080/10106049.2019.1597390. [ Links ]
Perry, K.I., Wallin, K.F., Wenzel, J.W. & Herms, D.A., 2017, 'Characterizing movement of ground-dwelling arthropods with a novel mark-capture method using fluorescent powder', Journal of Insect Behavior, 30, 32-47, https://doi.org/10.1007/s10905-017-9598-0. [ Links ]
Picker, M., Griffiths, C. & Weaving, A., 2019, Field guide to insects of South Africa, Penguin Random House South Africa, Cape Town. [ Links ]
R Development Core Team, 2021 R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, https://www.R-project.org. [ Links ]
Reihart, R.W., Angelos, K.P., Gawkins, K.M., Hurst, S.E., Montelongo, D.C., Laws, A.N., Pennings, S.C. & Prather, C.M., 2021, 'Crazy ants craving calcium: macronutrients and micronutrients can limit and stress an invaded grassland brown food web', Ecology, 102(2), e03263, https://doi.org/10.1002/ecy.3263. [ Links ]
Rivas-Arancibia, S.P., Carrillo-Ruiz, H., Arce, A.B., Figueroa-Castro, D.M. & Andrés-Hernández, A.R., 2014, 'Effect of disturbance on ant community in a semiarid region of central Mexico', Applied Ecology and Environmental Research, 12(3), 703 - 716, https://doi.org/10.15666/aeer/1203_703716. [ Links ]
Rochon, J., Gondan, M. & Kieser, M., 2012, 'To test or not to test: preliminary assessment of normality when comparing two independent samples', BMC Medical Research Methodology, 12, https://doi.org/10.1186/1471-2288-12-81. [ Links ]
Rosa, M.G., Brescovit, A.D., Baretta, C.R.D.M., Santos, J.C.P., De Oliveira Filho, L.C.I. & Baretta, D., 2019, 'Diversity of soil spiders in land use and management systems in Santa Catarina, Brazil', Biota Neotropica, 19(2), https://doi.org/10.1590/1676-0611-BN-2018-0619. [ Links ]
Samways, M.J., McGeoch, M.A. & New, T.R., 2010, Insect conservation: a handbook of approaches and methods, Oxford University Press, New York. [ Links ]
Savage, A.M., Youngsteadt, E., Ernst, A.F., Powers, S.A., Dunn, R.R. & Frank, S.D., 2018, 'Homogenizing an urban habitat mosaic: arthropod diversity declines in New York City parks after Super Storm Sandy', Ecological Applications, 28(1), 225-236, https://doi.org/10.1002/eap.1643. [ Links ]
Sayer, E.J., Sutcliffe, L.M.E., Ross, R.I.C. & Tanner, E.V.J., 2010, 'Arthropod abundance and diversity in a lowland tropical forest floor in Panama: the role of habitat space vs. nutrient concentrations', Biotropica, 42(2), 194-200, https://doi.org/10.1111/j.1744-7429.2009.00576.x. [ Links ]
Silveira, J.M., Barlow, J., Louzada, J. & Moutinho, P., 2010, 'Factors affecting the abundance of leaf-litter arthropods in unburned and thrice-burned seasonally-dry Amazonian forests' PLoS One, 5, https://doi.org/10.1371/journal.pone.0012877. [ Links ]
Smit, A.M. & Van Aarde, R.J., 2001, 'The influence of millipedes on selected soil elements: a microcosm study on three species occurring on coastal sand dunes', Functional Ecology, 15(1), 51-59, https://doi.org/10.1046/j.1365-2435.2001.00493.x. [ Links ]
Sonter, L.J., Ali, S.H. & Watson, J.E.M., 2018, 'Mining and biodiversity: key issues and research needs in conservation science', Proceedings of the Royal Society B 285, https://doi.org/10.1098/rspb.2018.1926. [ Links ]
Swart, R.C., Pryke, J.S. & Roets, F., 2018, 'Arthropod assemblages deep in natural forests show different responses to surrounding land use', Biodiversity and Conservation, 27, 583-606, https://doi.org/10.1007/s10531-017-1451-4. [ Links ]
Thanigaivel, S., Vickram, S., Dey, N., Jeyanthi, P., Subbaiya, R., Kim, W., Govarthanan, M. & Karmegam, N., 2023, 'Ecological disturbances and abundance of anthropogenic pollutants in the aquatic ecosystem: critical review of impact assessment on the aquatic animals', Chemosphere, 313, https://doi.org/10.1016/j.chemosphere.2022.137475. [ Links ]
Tonello, M.S., Korchagin, J. & Bortoluzzi, E.C., 2021, 'Environmental agate mining impacts and potential use of agate residue in rangeland', Journal of Cleaner Production, 280, https://doi.org/10.1016/j.jclepro.2020.124263. [ Links ]
Tropek, R., Kadlec, T., Karesova, P., Spitzer L., Kocarek, P., Malenovsky, I., Banar, P., Tuf, I.H., Hejda, M. & Konvicka, M., 2010, 'Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants', Journal of Applied Ecology, 47(1), 139-147, https://doi.org/10.1111/j.1365-2664.2009.01746.x. [ Links ]
Uhey, D.A., Riskas, H.L., Smith, A.D. & Hofstetter, R.W., 2020, 'Ground-dwelling arthropods of pinyon-juniper woodlands: arthropod community patterns are driven by climate and overall plant productivity, not host tree species', PLoS One, 15, e0238219, https://doi.org/10.1371/journal.pone.0238219. [ Links ]
Van der Wal, A., Geerts, R.H.E.M., Korevaar, H., Schouten, A.J., Jagers op Akkerhuis, G.A.J.M., Rutgers, M. & Mulder, C., 2009, 'Dissimilar response of plant and soil biota communities to long-term nutrient addition in grasslands', Biology and Fertility of Soils, 45, 663-667, https://doi.org/10.1007/s00374-009-0371-1. [ Links ]
Wagner, D., Brown, M.J.F. & Gordon, D.M., 1997, 'Harvester ant nests, soil biota and soil chemistry', Oecologia, 112(2), 232-236, https://doi.org/10.1007/s004420050305. [ Links ]
Wang, D., Zheng, L., Ren, M., Li, C., Dong, X., Wei, X., Zhou, W. & Cui, J., 2022, 'Zinc in soil reflecting the intensive coal mining activities: evidence from stable zinc isotopes analysis', Ecotoxicology and Environmental Safety, 239, https://doi.org/10.1016/j.ecoenv.2022.113669. [ Links ]
With, K.A. & Pavuk, D.M., 2012, 'Direct versus indirect effects of habitat fragmentation on community patterns in experimental landscapes', Oecologia, 170(2), 517-528, https://doi.org/10.1007/s00442-012-2325-9. [ Links ]
White, P.J. & Broadley, M.R., 2003. 'Calcium in plants', Annals of Botany, 92(4), 487-511. https://doi.org/10.1093/aob/mcg164. [ Links ]
White, R.E., 1998, The Beetles of North America, Houghton Mifflin Harcourt, Boston. [ Links ]
Yan, B. & Hou, Y., 2018, 'Effect of soil magnesium on plants: a review', IOP Conference Series: Earth and Environmental Science, 170, 022168, https://doi.org/10.1088/1755-1315/170/2/022168. [ Links ]
Yekwayo, I., Prypke, J.S., Roets, F. & Samways, M.J., 2016, 'Surrounding vegetation matters for arthropods of small, natural patches of indigenous forest', Insect Conservation and Diversity, 9(3), 224-235, https://doi.org/10.1111/icad.12160. [ Links ]
Yekwayo, I., Pryke, J.S., Roets, F. & Samways, M.J., 2017, 'Responses of ground living arthropods to landscape contrast and context in a forest-grassland mosaic', Biodiversity and Conservation, 26, 631-651, https://doi.org/10.1007/s10531-016-1262-z. [ Links ]
Zhao, J., Dong, Y., Xie, X., Li, X., Zhang, X. & Shen, X., 2011, 'Effect of annual variation in soil pH on available soil nutrients in pear orchards', Acta Ecologica Sinica, 31(4), 212-216. https://doi.org/10.1016/j.chnaes.2011.04.001. [ Links ]
Zhao, K., Jing, X., Sanders, N.J., Chen, L., Shi, Y., Flynn, D.F.B., Wang, Y., Chu, H., Liang, W. & He, J.S., 2017, 'On the controls of abundance for soil-dwelling organisms on the Tibetan Plateau', Ecosphere 8(7), e01901, https://doi.org/10.1002/ecs2.1901. [ Links ]
Zulka, K.P., 2013, 'Effects of gravel mining on the surface-active arthropod fauna of ephemeral gravel-bed stream valleys in the National Park Gesäuse (Styria, Austria)', 5th Symposium for research in protected areas, Mittersill, 10-12 June 2013. [ Links ]
 Correspondence:
Correspondence:
Inam Yekwayo
e-mail: iyekwayo@wsu.ac.za
Submitted: 13 December 2022
Accepted: 2 August 2023
Published: 30 November 2023
Supplementary Material














