Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.51 n.1 Pretoria 2021
http://dx.doi.org/10.38201/btha.abc.v51.i1.9
Chlorophyll a fluorescence measurements of W. mirabi-lis plants were taken during winter (July) with a Handy Pea (Plant Efficiency Analyzer) fluorometer during the night. Measurements were taken one hour after sunset and continued to approximately midnight. Such dark adaption was done to ensure that all reaction centres were open. Ten fluorescence measurements were taken at different spots on each plant within the first 10 cm from the leaf base. During the time of the measurements, the night temperature was around 18°C. The Handy Pea was calibrated to produce pulses of light with an intensity of 3 445 ^mol mol-1 and a gain of 1. These light pulses each had a duration of 1 second at a wavelength of 650 nm.
The chlorophyll fluorescence induction curve has specific inflection points named O, K, J, I and P plotted on a logarithmic time scale (OJIP transient). Typically, stress will influence the shape of the OJIP transients by causing a shift in the induction curve. Assessing shifts in the induction curve provides information regarding the photosynthetic potential of the plant and, ultimately, plant health. The OJIP transient section between steps J and P is known as the thermal phase (or the multiple turn-over phase). This phase represents the reduction of the electron transport chain. The step between J and I is associated with the reduction of the PQ-pool and the I to P step with electron flow through photosystem I (Stirbet & Govindjee 2011).
The performance index (PIABS) is a widely used JIP-test parameter that provides quantitative information about the physiological state of plants and vitality. PIABS provides information about the potential for energy conservation from light absorption to the reduction of intersystem electron acceptors (Strasser et al. 2004). It is a function of its three partial parameters: the density of active reaction centres per chlorophyll (yRC/(1-yRC)), the efficiency of electron movement by trapped excitation into electron transport chain ((ppO/(1-(ppO)) and the probability that the reaction centres will trap an absorbed photon (i|/Eo/(1-i|/Eo)) (Strasser et al. 2000). If a stress condition influences any of these partial parameters, the stress will influence the PIABS values and reflect the current state of photosyn-thetic performance (Strasser et al. 2004).
The fluorescence data captured with the Handy Pea was analysed with PEA Plus 1.140 software. The River Channel was selected as the reference site as the plants represented at this site were visually larger and appeared healthier compared to the other two sites. The translation of the fluorescence data to biophysical parameters was done according to Strasser et al. (2004). Data were subjected to the Shapiro-Wilk test and if data conformed to the assumptions of normality, a one-way ANOVA was run for each parameter in Statistica v13, Dell Inc. (2016).
Results
Typical chlorophyll a fluorescence induction curves of dark-adapted leaves of W. mirabilis plants were plotted for the Campsite, River Channel and Zone 6 catchments (Figure 2). The time frame between steps O and J (also referred to as the single turn-over phase or the photochemical phase) provides information about the antenna size and the connectivity between photosystem II reaction centres (Strasser et al. 2004). When the induction curves from the different catchments were compared to one another during this time frame, there were no apparent shifts in the induction curve from any of the catchments (Figure 2). A shift in the shape of the Campsite induction curve was observed between steps I and P. The rise in the fluorescence transient, especially after the I-step, was the lowest at Campsite. This would imply that the electron flow between photosystem II and photosystem I was less efficient (Figure 2).
W. mirabilis plants located at Campsite had significantly lower PIABS (P < 0.05) values when compared to the other catchments (Figure 3). The lower PIABS values of Campsite suggests that these W. mirabilis plants are in a less optimal condition. By normalising the JIP parameters of Campsite and Zone 6 to River Channel (references site), differences among the catchments were emphasised. From the spider plot it was clear that all of the parameters that comprise the PIABS values for Campsite were all lower than those of River Channel and Zone 6 (Figure 4). The density of active reaction centres (yRC/(1- YRC)), the potential to create a charge separation (φρ(_/(1- φ )) and the potential to transport electrons between pSiI and PSI (v|/Eo/(1-v|/Eo)) were all lower at Campsite (Figure 4).
The total performance index, PItotal, is the product of the PIABS and the probability that an electron can move from a reduced intersystem electron acceptor to the PSI end-electron acceptors (Tsimilli-Michael & Strasser 2008). The PI index is, therefore, very closely related to the plants' overall growth and health. The PI values of the welwitschia plants growing at Campsite were also significantly lower (P < 0.05). The only difference between the PItotal and the PIabs is that for calculating the PItotal index, the reduction of the end electron acceptors (5Ro/(1-5Ro)) is included. Overall the spider plot (Figure 4) clearly shows that the photosynthetic potential of the W. mirabilis plants at Campsite was less efficient compared to the other two catchments.
Discussion
Monitoring the health status of W. mirabilis plants is critical for early detection of the impact of land-use change. Chlorophyll a fluorescence measurements, which were taken before the onset of mining activi-tiesm, can now serve as a baseline for future monitoring
(Kalaji et al. 2016). The vitality statuses of plants before mining activities is indicated by differences in the photosynthetic potential between W. mirabilis plants located within different catchment areas in the same locality. The maximum photosynthetic quantum yield of W. mirabilis plants located at Campsite was lower than River Channel and Zone 6, indicating a less optimal health condition when compared to the latter two.
The Campsite catchment has a higher elevation than the other catchments resulting in lower water accumulation, as water accumulates along the flow paths, which is influenced by the topography (Fan et al. 2020). This lower water accumulation at Campsite will lower the photosynthetic potential of the W. mirabilis plants. Topographic features such as elevation and slope may also change vegetation exposure to wind and solar radiation, contributing to a decrease in the photosynthetic potential (Mikita & Klimánek 2010). Because this study was conducted during winter, episodic rainfall did not influence the measurements. Incoming fog from the Atlantic Ocean might influence the photosynthetic potential of plants and has to be acknowledged, but the selected welwitschia plants for this study were chosen from outside the reach of the incoming fog.
For all practical reasons, the PItotal performance index or any partial parameters could have been used (Kalaji et al. 2016). To optimise the value of the JIP-test, annual readings should be taken on the same plants and any changes in the JIP parameters should be noted. It is recommended that several measurements be taken throughout the year. The data from this study represent the environmental conditions during the winter and if this same investigation was carried out during the summer, different PIABS values would be obtained, but the trend should remain the same (Janssen & Hasselt 1994).
Considering the planned change in anthropogenic activities, together with natural stressors on the fringes of the Welwitschia Plains, it is imperative to detect changes in the health status before the onset of visible stress symptoms. This early detection of plant stress will prompt for management actions to prevent populations from being adversely affected (Chaerle & Van Der Straeten 2000). Therefore, the chlorophyll fluorescence parameters analysis can be a very informative tool in ecological surveys (Kalaji et al. 2016) by providing explanations on the physiological behaviour of W. mirabilis plants in response to its changing environment. We suggest that long-term monitoring studies integrating potential drivers and responses be conducted to understand the plant health of W. mirabilis across the landscape. This study has established a baseline that can be used to develop a protocol to monitor the plant physiological status and the possible management strategies for mines and other developments that may have adverse impacts on the W. mirabilis population. Besides that, the findings may also aid restoration and rehabilitation measures such as transplantation and re-introduction of this species by understanding its current functional health status across the landscape over time.
Conclusion
Chlorophyll a fluorescence measuring techniques have high potential to investigate plant health in situ in longterm monitoring. Our study was a preliminary one, conducted over only a short period. Considering the longevity of welwitschia plants and the urgent need to develop a thorough understanding of how the species reacts to different stressors created by land-use change, longer-term studies should be conducted to understand the in situ spatial and temporal patterns of the species' health. With increasing mining activities on the fringes of the Welwitschia Plains and the potential threat that these anthropogenic activities pose to the welwitschia population, continued monitoring is vital.
Acknowledgments
The authors of this paper would like to thank the National Research Foundation (NRF) in South Africa and the National Commission on Research, Science and Technology (NCRST) of Namibia for funding this project. Also, our appreciation to the Gobabeb Research and Training Centre, Namibia, for organising the permits to conduct research within the Welwitsch-ia Plains and to the Gobabeb volunteers who assisted with the collection of data.
Authors' contributions
JMB planned and coordinated the study, collected field data, conducted data analyses and wrote the manuscript. HC collected field data, conducted data analyses and contributed to the writing of the manuscript. TS collected field data and contributed to the analysis & interpretation of the data.
Disclaimer
The views expressed in the submitted article are our own and not an official position of the institution or funder.
References
Busotti, F., Desotgiu, R., Pollastrini, M. & Cascio, C., 2010, 'The JIP test: a tool to screen the capacity of plant adaptation to climate change', Scandanavian Journal of Forest Research, 25(8), 43-50, https://doi.org/10.1080/02827581.2010.485777. [ Links ]
Chaerle, L. & Van Der Straeten, D., 2000, 'Imaging techniques and the early detection of plant stress', Trends in Plant Science, 5(11), 495-501, https://doi.org/10.1016/S1360-1385(00)01781-7. [ Links ]
Cooper-Driver, G., 1994, 'Welwitschia mirabilis - a dream come true', Arnoldia, 54(2), 2-10. [ Links ]
Henschel, J. & Seely, M.K., 2000, 'Long-term growth patterns of Welwitschia mirabilis, as long-lived plant of the Namib Desert', Plant Ecology, 150(1-2), 7-26, https://doi.org/10.1023/A:1026512608982. [ Links ]
Fan, J., Xu, Y., Ge, H. & Yang, W., 2020, 'Vegetation growth variation in relation to topography in Horqin Sandy Land', Ecological Indicators, 113, 106-215. [ Links ]
Humavindu, M. & Stage, J., 2013, 'Key sectors of the Namib-ian economy', Journal of Economic Structures, 2(1), 1-15, https://doi.org/10.1186/2193-2409-2-1. [ Links ]
Janssen, L.H. & Hasselt, PR., 1994, 'Temperature effects on chlorophyll fluorescence induction in tomato', Journal of Plant Physiology, 144(2), 129-135. [ Links ]
Kalaji, H.M., Jajoo, A., Oukarroum, A., Brestic, M., Zivcak, M., Samborska, I.A. & Ladle, R.J., 2016, 'Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions', Acta Physiologiae Plantarum, 38(4), 102, https://doi.org/10.1007/s11738-016-2113-y. [ Links ]
Mikita, T. & Klimánek, M., 2010, 'Topographic exposure and its practical applications', Jounal of Landscape Ecology, 3(1), 42-51. [ Links ]
Schulze, E.D., Ziegler, H. & Stichler, W., 1976, 'Environmental control of Crassulacean Acid Metabolism in Welwitsch-ia mirabilis Hook. Fil. in in its range of natural distribution in the Namib desert', Oecologia, 24, 323-334. [ Links ]
Shanyengana, E.S., Henschel, J.R., Seely, M.K. & Sanderson, R.D., 2002, 'Exploring fog as a supplementary water source in Namibia', Atmospheric Research, 64(1), 251-259, DOI: 10.1016/S0169-8095(02)00096-0. [ Links ]
Stirbet, A., 2011, 'On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basic applications of the OJIP fluorescent transient', Journal of Photochemistry & Photobiology B: Biology, 104, 236-257, DOI: 10.1016/j.jphotobiol.2010.12.010. [ Links ]
Strasser, R.J., Srivastava, A. & Tsimilli-Michael, M., 2000, 'The fluorescence transient as a tool to characterize and screen photosynthetic samples', in M. Yunus, U. Pathre & P. Mohanty (eds), Probing photosynthesis: Mechanisms, regulation and adaptation, London: Taylor & Francis, pp. 445-483. [ Links ]
Strasser, R.J., Tsimilli-Michael, M. & Srivastava, A., 2004, 'Analysis of the chlorophyll a Fluorescence Transient', in G.C. Papageorgiou & Govindjee (eds), Chlorophyll a fluorescence. A signature of photosynthesis. Advances in Photosynthesis and Respiration, vol 19. Dordrecht: Springer, p. 818. [ Links ]
Tsimilli-Michael, M. & Strasser, R.J., 2008, 'In vivo assessment of stress impact on plant's vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants', in A. Varma (ed.), Mycorrhiza. Berlin: Springer. [ Links ]
Veste, M. & Herppich, W.B., 2008, 'Welwitschia mirabilis -Eine ökophysiologische Betrachtung', Naturwissenschaftliche Rundschau, 61, 620-624. [ Links ]
von Willert, D.J., Armbrüster, N., Drees, T. & Zaborowski, M., 2005, 'Welwitschia mirabilis: CAM or not CAM - what is the answer?', Functional Plant Biology, 32(5), 389-395, DOI: 10.1071/FP01241. [ Links ]
World Heritage Convention, 2002. Welwitschia plains. Available at: http://whc.unesco.org/en/tentativelists/1747/ (Accessed: 9 September 2015). [ Links ]
 Correspondence:
Correspondence:
Dr Jacques Berner
jacques.berner@nwu.ac.za
Submitted: 24 September 2019
Accepted: 15 October 2020
Published: 24 February 2021
SHORT COMMUNICATION
Drought tolerant forb flora of a semi-arid protected savanna in the Lowveld of South Africa
H. van Coller; J. Klem; F. Siebert
Unit for Environmental Sciences and Management, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa
ABSTRACT
BACKGROUND: Increased frequency and intensity of droughts related to climate change are predicted to induce pressure on herbaceous communities. Considering that forbs contribute significantly to savanna ecosystem resilience, we investigated forb communities of a protected semi-arid savanna during an extensive drought.
OBJECTIVE: We identified drought-tolerant species with their related functional traits.
RESULTS: Drought-tolerant forb flora comprised of several plant families and species with overlapping traits, of which the ability to resprout was related to perennials, whereas succulence and prostrate growth form were typical annual forb dominance traits.
CONCLUSION: Results highlight the functional importance of forbs and their resilience to drought events in protected areas.
Keywords: resprouting; herbaceous communities; functional traits; resilience; climate change.
Introduction
Predicted increasing drought intensity and frequency, combined with higher average temperatures due to global climate change, are threatening biodiversity, and therefore the stability, functioning and sustainability of terrestrial ecosystems (Barros et al. 2018). Drought is a common phenomenon in semi-arid rangelands (Vetter 2009) and has been shown to cause rapid and lasting effects on vegetation dynamics and ultimately ecosystem function and services (Barros et al. 2018; Ploughe et al. 2019).
Depending on intensity, droughts can cause shifts in plant species assemblages, leading to the establishment of different plant communities (Junk et al. 2018). In herbaceous layers of semi-arid savannas, these communities are composed mainly of annual grasses and both annual and perennial forbs (O'Connor 1998; Buitenwerf et al. 2011). In the savanna context, the term 'forb' is used to classify anything other than trees, shrubs and grasses, which has led to a poor functional definition of this life form. For the purpose of this study, we will define forbs as non-graminoid vascular plants with limited woody tissue and with perennating buds at or below soil surface. Drought episodes tend to favour forbs, since they possess a variety of drought-tolerant traits such as underground storage organs (Siebert et al. 2019) associated with persistent bud banks and viable seed banks (Siebert & Dreber 2019). Despite being associated with savanna land degradation and therefore perceived as being an undesirable functional group by land managers (Fynn & O'Connor 2000; Tes-sema et al. 2011), forbs are important through providing ecosystem functions (i.e. forage stability) during stressed conditions, and functional redundancy to absorb disturbances such as sustained grazing and droughts (Van Coller et al. 2018).
Forbs are an important source of nutritious forage and may constitute an important part of ungulate diets at certain times of the year (Du Toit 2003; Van Der Merwe & Marshal 2012). Moreover, forbs contribute significantly to the biodiversity of savanna and grassland systems (Buitenwerf et al. 2011; Siebert & Scogings 2015), which are functionally diverse, suggesting a stronger resilience to different environmental conditions (Turner & Knapp 1996; Van Coller et al. 2018). Forb ecology research in dry African savannas generally report on forb responses at the level of functional group rather than species level. Furthermore, variations in forb functional traits defining plant strategies for regeneration and survival in adaptation to climate extremes such as droughts, remain understudied (Siebert & Dreber 2019).
Below-average rainfall in the Central Lowveld of South Africa was recorded for two consecutive years (2015 and 2016) (Swemmer et al. 2018). Using data collected during this time, we sought to identify specific drought tolerant forb species and their respective functional traits in a semi-arid African savanna. In doing so, we aimed to enhance knowledge of the attributes that allow these forb species to persist during droughts and potential functions that they fulfil under such environmental conditions.
Materials and Methods
Forb communities were studied in the semi-arid savanna of the greater Kruger National Park (KNP). Protected areas are not exempt from natural disasters, such as drought. They therefore provide valuable natural experimental settings where spatial heterogeneity and ecological responses function under natural drivers (Pickett et al. 2003). These areas host a variety of indigenous wildlife including mixed feeders (e.g. elephants [Lox-odonta africana (Blumenbach, 1797)]; impala [Aepy-ceros melampus (Lichtenstein, 1812)]), browsers (e.g. greater kudu [Tragelaphus strepsiceros (Pallas, 1766)]; bushbuck [Tragelaphus sylvaticus (Sparrman, 1780)]), and grazers (e.g. blue wildebeest [Connochaetes tau-rinus (Burchell, 1823)]; plains zebra [Equus quagga (Boddaert, 1785)], amongst others (Van der Waal et al. 2011; Scogings et al. 2012).
Field surveys were undertaken at two sites of similar geology (i.e. granite and gneiss), but different soil nutrient statuses (i.e. nutrient-rich sodic soil versus nutrient-poor sandy soil). Floristic and functional trait data were collected from 48 plots of 1 m2 (18 plots within the nutrient-rich site and 30 plots within the nutrient-poor site) during the usual rainy season (November-March) of the extensive drought of 2015/2016. In the KNP (i.e. nutrient-rich site), total annual rainfall was 200 mm below the mean annual rainfall for the area (Van Coller et al. 2018), while at the nutrient-poor site in Timbavati Private Nature Reserve (TPNR) it was ~330 mm below the long-term average (Kaschula et al. 2005). Within each plot, forbs were identified up to species level and all individuals counted. Frequency per species was calculated with respect to all recorded species in each respective site. Frequency is considered a stable variable for the abundance of an individual species (O'Connor 2015). Frequency measures (%) were used to identify forb species most commonly observed in the study sites. Only forb species with a frequency > 1 are discussed. Functional traits were assigned to forb species (Cornelissen et al. 2003; Germishuizen & Meyer 2003) based on the potential contribution to the functioning of semi-arid protected areas (i.e. palatability, life history and nitrogen-fixing ability), as well as the ability to tolerate conditions related to drought and herbivory (growth form, life history, resprouting capacity and succulence).
Results and Discussion
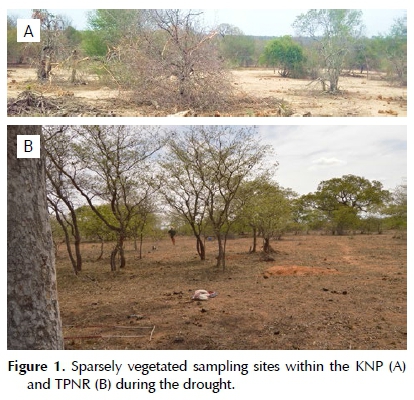
Herbaceous productivity is strongly affected by rainfall, and generally reveals marked deterioration in response to drought conditions (Figure 1) (Fynn & O'Connor 2000; O'Connor 2015). Despite this, frequency measures revealed a total of 31 forb species among the two study sites. A mean number of six and four forb species was recorded per plot (1 m2) in the nutrient-rich and nutrient-poor sites respectively. The number of forb species per plot recorded in the nutrient-rich site ranged from one to 17, while a lower range (0-7) of forb species were recorded for plots in the nutrient-poor site.
Plant families that comprised most of the frequent taxa during the drought included Acanthaceae and Amaranthaceae in the nutrient-rich site, and Faba-ceae and Boraginaceae in the nutrient-poor site (Table 1). Prevalence of the Fabaceae is in accordance with Wagner et al. (2016) who reported that nitrogen-fixing herbaceous legumes from the Fabaceae may increase in abundance after disturbances in dry savanna rangelands, and also in disturbed grasslands (Muller et al. 2021). Nitrogen-fixing ability is a trait generally associated with ecosystems with low nutrient availability (Cornelissen et al. 2003), explaining the high frequency of Chamae-crista mimosoides (L.) Greene in the nutrient-poor site (Table 1). Over 50% of the most frequent forbs were annuals (Table 1). Annual forbs have been reported to form a major component of soil seed banks, especially under heavy grazing (O'Connor 1991; Tessema et al. 2016), potentially enabling them to respond and establish rapidly when conditions become favourable (e.g. smaller rainfall events interrupting extensive droughts). The occurrence of bare soil caused by herbivores and drought is known to facilitate the colonisation of prostrate forb species (Burkepile et al. 2016). Blepharis inte-grifolia (L.f.) E.Mey. ex Schinz, a palatable, perennial and low-growing forb is known to form patches of continuous groundcover or 'browsing lawns' in heavily utilised sodic bottomlands (Siebert & Scogings 2015), whilst the prostrate-growing annual Gisekia africana (Lour.) Kuntze revealed the highest frequency in the nutrient-poor site (Table 1). Persistence of these species is therefore likely attributed to the positioning of perennating tissue at or close to the soil surface, since fewer species could have buds far above the soil surface during harsher climatic conditions such as drought (Cornelissen et al. 2003). Moreover, erect growing plant species with their peren-nating buds situated above the soil surface are especially susceptible to trampling, heavy grazing and exposure to extreme heat conditions, whereas prostrate-growing species are avoidant by retaining buds and leaf material close to the soil surface (Cornelissen et al. 2003). A prostrate growth form in forbs could therefore be considered an important resistance trait against drought and grazing in the protected Lowveld savannas of South Africa.
The presence of annual forb species with a pioneer character in seed banks (Tessema et al. 2016) allows for their initial colonisation of bare soil (Siebert & Dre-ber 2019). Therefore, some of the most frequently observed forb species during the drought (i.e. Portu-laca kermesina N.E.Br. and P. hereroensis Schinz in the nutrient-rich site, and G. africana in the nutrient-poor site) were annuals (Table 1). Moreover, these species exhibited traits generally associated with grazing- and drought-tolerance (i.e. prostrate growth form and succulence) (Cornelissen et al. 2003). Although little is known about succulence as a drought-tolerant trait in forbs, the ability of these species to retain water in their leaves and stems during dry conditions, together with a prostrate growth form to avoid and tolerate herbivory, possibly favoured their survival when subjected to herbivore utilisation in dry, hot conditions. Annual forbs exhibiting this combination of traits therefore make up an important component of the forb flora of semi-arid protected areas, especially during a drought.
The majority of drought-tolerant perennial forbs in this study had the ability to resprout, either through buds located at or near the soil surface, or belowground. Such a disturbance-tolerant trait is well-known for trees and shrubs, but our understanding of regeneration from buds in forbs is unknown and requires further investigation (Siebert & Dreber 2019).
Over half of the frequent forb species recorded during the drought were palatable (Table 1). This reinforces the functional importance of the forb component through their ability to provide important ecosystem functions, such as forage stability during stressed conditions, and functional redundancy enabling them to absorb disturbances such as sustained grazing and drought (Van Col-ler et al. 2018).
Conclusion
Despite anticipated deterioration of the herbaceous layer during droughts, forbs have the ability to withstand such disturbances through species-specific adaptions. Numerous forb species were able to persist amidst the abnormal hot and dry conditions, while providing the ecosystem with important functions and services, such as forage stability. Plant strategies for survival and regeneration during drought conditions are species- and family specific, which may vary across ecosystem types. Furthermore, drought-adaptations were also specific for life history groups, as annuals displayed strategies to survive after emergence (e.g. succulence and prostrate growth form), whilst the majority of perennial forbs had the ability to resprout from a persistent bud bank. As protected areas aim to conserve biodiversity, provide forage security for wildlife and to maintain ecosystem resilience, this study demonstrates that forbs contribute to these at plant taxonomic and functional trait levels.
Authors' contributions
HvC (North-West University) collected vegetation data from the Nkuhlu exclosures at the Kruger National Park (KNP), analyzed and reported data and wrote the manuscript, whilst JK (North-West University) collected data from Timbavati Private Nature Reserve (TPNR). FS (North-West University) was the project leader, promoter and supervisor to HvC and JK respectively, and was responsible for project design, data collection, reporting and the writing of the manuscript.
Disclaimer
The authors declare that the work presented, and views expressed in this submitted article is their own and is not an official position of the institution or funder. The authors furthermore declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Source(s) of support
South African National Parks, National Research Foundation, North-West University.
References
Barros, C., Thuiller, W. & Münkemüller, T., 2018, 'Drought effects on the stability of forest-grassland ecotones under gradual climate change', PLoS ONE, 13(10), 1-18. https://doi.org/10.1371/journal.pone.0206138. [ Links ]
Buitenwerf, R., Swemmer, A.M. & Peel, M.J.S., 2011, 'Longterm dynamics of herbaceous vegetation structure and composition in two African savanna reserves', Journal of Applied Ecology, 48(1), 238-246. https://doi.org/10.1111/j.1365-2664.2010.01895.x. [ Links ]
Burkepile, D.E., Thompson, D.I., Fynn, R.W.S., Koerner, S.E., Eby, S., Govender, N., Hagenah, N., Lemoine, N.P, Match-ett, K.J., Wilcox, K.R., Collins, S.L., Kirkman, K.P., Knapp, A.K. & Smith, M.D., 2016, 'Fire frequency drives habitat selection by a diverse herbivore guild impacting top-down control of plant communities in an African savanna', Oikos, 125, 1636-1646. https://doi.org/10.1111/oik.02987. [ Links ]
Cornelissen, J.H.C., Lavorel, S., Garnier, E., Díaz, S., Buchmann, N., Gurvich, D.E., Reich, P.B., ter Steege, H., Morgan, H.D., van der Heijden, M.G.A., Pauses, J.G. & Poorter, H., 2003, 'A handbook of protocols for standardised and easy measurement of plant functional traits worldwide', Australian Journal of Botany, 51(4), 335. https://doi.org/10.1071/bt02124. [ Links ]
Du Toit, J.T., 2003, 'Large herbivores and Savanna heterogeneity', in du Toit, J.T., Biggs, H.C. & Rogers, K.H. (eds.), The Kruger Experience: Ecology and Management of Savanna Heterogeneity, pp. 292-309, Washington, DC: Island Press. [ Links ]
Fynn, R.W.S. & O'Connor, T.G., 2000, 'Effect of stocking rate and rainfall on rangeland dynamics and cattle performance in a semi-arid savanna, South Africa', Journal of Applied Ecology, 37(3), 491-507. https://doi.org/10.1046/j.1365-2664.2000.00513.x. [ Links ]
Germishuizen, G. & Meyer, N.L. (eds.), 2003, 'Plants of southern Africa: an annotated checklist', Strelitzia 14, Pretoria: National Botanical Institute. [ Links ]
Junk, W.J., Piedade, M.T.F., Cunha, C.N. da, Wittmann, F., & Schöngart, J., 2018, 'Macrohabitat studies in large Brazilian floodplains to support sustainable development in the face of climate change', Ecohydrology and Hydrobi-ology, 18(4), 334-344. https://doi.org/10.1016/j.ecohyd.2018.11.007. [ Links ]
Kaschula, S.A., Twine, W.E., & Scholes, M.C., 2005, 'Coppice harvesting of fuelwood species on a South African common: Utilizing scientific and indigenous knowledge in Community Based Natural Resource Management', Human Ecology, 33, 387-418. https://doi.org/10.1007/s10745-005-4144-7 [ Links ]
Muller, M., Siebert, S.J., Ntloko, B.R. & Siebert, F. 2021, 'A floristic assessment of grassland diversity loss in South Africa', Bothalia 51(1): 147-155 (hardcopy); 1-9 (online) [this issue]. [ Links ]
O'Connor, T.G., 1991, 'Influence of rainfall and grazing on the compositional change of the herbaceous layer in the savanna regions of southern Africa', Journal of the Grassland Society of southern Africa, 8, 103-109. https://doi.org/10.1080/02566702.1991.9648273. [ Links ]
O'Connor, T.G., 1998, 'Impact of sustained drought on a semi-arid Colophospermum mopane, African Journal of Range and Forage Science, 15(3), 83-91. https://doi.org/10.1080/10220119.1998.9647948. [ Links ]
O'Connor, T.G., 2015, 'Long-term response of an herbaceous sward to reduced grazing pressure and rainfall variability in a semi-arid South African savanna', African Journal of Range and Forage Science, 32(4), 261-270. https://doi.org/10.2989/10220119.2015.1015052. [ Links ]
Pickett, S.T.A., Cadenasso, M.L. & Benning, T.L., 2003, 'Biotic and abiotic variability as key determinants of savanna heterogeneity at multiple spatiotemporal scales', in du Toit, J.T., Biggs, H.C. & Rogers, K.H. (eds.), The Kruger Experience: Ecology and management of savanna heterogeneity, pp. 22-40, Island Press, Washington, DC. [ Links ]
Ploughe, L.W., Jacobs, E.M., Frank, G.S., Greenler, S.M., Smith, M.D., & Dukes, J.S., 2019, 'Community Response to Extreme Drought (CRED): a framework for drought-induced shifts in plant-plant interactions', New Phytologist, 222, 52-69. http://doi.org/10.1111/nph.15595 [ Links ]
Scogings, PF., Johansson, T., Hjaltén, J. & Kruger, J., 2012, 'Responses of woody vegetation to exclusion of large herbivores in semi-arid savannas', Austral Ecology, 37(1), 56-66. https://doi.org/10.1111/j.1442-9993.2011.02249. [ Links ]
Scogings, PF., Hattas, D., Skarpe, C., Hjaltén, J., Dziba, L., Zobo-lo, A., & Rooke, T., 2015, 'Seasonal variations in nutrients and secondary metabolites in semi-arid savannas depend on year and species', Journal of Arid Environments, 114, 54-61. https://doi.org/10.1016/j.jaridenv.2014.11.003 [ Links ]
Siebert, F. & Scogings, PF., 2015, 'Browsing intensity of herbaceous forbs across a semi-arid savanna catenal sequence', South African Journal of Botany, 100, 69-74. https://doi.org/10.1016/j.sajb.2015.05.007. [ Links ]
Siebert, F. & Dreber, N., 2019, 'Forb ecology research in dry African savannas: Knowledge, gaps, and future perspectives', Ecology and Evolution, 9, 7875-7891. https://doi.org/10.1002/ece3.5307. [ Links ]
Siebert, F., Bombo, A.B., Archibald, S., Greve, M., & Fidelis, A., 2019, 'Introducing bud bank and below-ground plant organ research to South Africa: Report on a workshop and the way forward', South African Journal of Science, 115(11/12), Art.#6803. https://doi.org/10.17159/sajs.2019/6803. [ Links ]
Swemmer, A.M., Bond, W.J., Donaldson, J., Hempson, G.P., Malherbe, J. & Smit, I., 2018, 'The ecology of drought - a workshop report', South African Journal of Science, 114(9/10), 9-11. https://doi.org/10.17159/sajs.2018/5098. [ Links ]
Tessema, Z.K., de Boer, W.F., Baars, R.M.T. & Prins, H.H.T., 2011, 'Changes in soil nutrients, vegetation structure and herbaceous biomass in response to grazing in a semi-arid savanna of Ethiopia', Journal of Arid Environments, 75(7), 662-670. https://doi.org/10.1016/j.jaridenv.2011.02.004. [ Links ]
Tessema, Z.K., de Boer, W.F. and Prins, H.H.T., 2016, 'Changes in grass plant populations and temporal soil seed bank dynamics in a semi-arid African savanna: Implications for restoration', Journal of Environmental Management, 182, 166-175. https://doi.org/10.1016/j.jen-vman.2016.07.057 [ Links ]
Turner, A.C.L. & Knapp, A.K., 2008, 'Responses of a C4 Grass and three C3 forbs to variation in nitrogen and light in Tall-grass Prairie, Ecological Society of America, 77(6), 17381749. http://www.jstor.org/stable/2265779. [ Links ]
Van Coller, H., Siebert, F., Scogings, PF., & Ellis, S., 2018, 'Herbaceous responses to herbivory, fire and rainfall variability differ between grasses and forbs', South African Journal of Botany, 119, 94-103. https://doi.org/10.1016/j.sajb.2018.08.024. [ Links ]
Van Der Merwe, J. & Marshal, J.P, 2012, 'Hierarchical resource selection by impala in a savanna environment', Austral Ecology, 37(3), 401-412. https://doi.org/10.1111/j.1442-9993.2011.02297.x. [ Links ]
Van der Waal, C., Kool, A., Meijer, S.S., Kohi, E., Heitkönig, I.M.A., de Beer, W.F., van Langevelde, F., Grant, R.C., Peel, M.J.D., Slowtow, R., de Knegt, H.J., Prins, H.H.T., & de Kroon, H., 2011, 'Large herbivores may alter vegetation structure of semi-arid savannas through soil nutrient mediation', Oecologia, 165(4), 1095-1107. https://doi.org/10.1007/s00442-010-1899-3. [ Links ]
Vetter, S., 2009, 'Drought, change and resilience in South Africa's arid and semi-arid rangelands.', South African Journal of Science, 105(1/2), 29-33. https://doi.org/10.1590/s0038-23532009000100017. [ Links ]
Wagner, T. C., Hane, S., Joubert, D.F. & Fischer, C., 2016, 'Herbaceous legume encroachment reduces grass productivity and density in arid rangelands', PLoS ONE, 11(11), 1-13. https://doi.org/10.1371/journal.pone.0166743. [ Links ]
 Correspondence:
Correspondence:
Dr H. van Coller
helga.vancoller@nwu.ac.za
Submitted: 20 September 2019
Accepted: 2 July 2020
Published: 24 February 2021
SHORT COMMUNICATION
A floristic assessment of grassland diversity loss in South Africa
M. MullerI; S.J. SiebertI; B.R. NtlokoII; F. SiebertI
IUnit for Environmental Sciences and Management, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa
IILetseng Diamonds, cnr Kingsway and Old School Road Maseru, P.O. Box 12508, Maseru 100 lesotho
ABSTRACT
BACKGROUND: Land-use effects on grassland flora are difficult to predict due to poor understanding of species losses caused by transformation.
OBJECTIVES: To determine changes in species diversity and composition by comparing transformed with untransformed grassland.
METHODS: Floristics of paired plots were sampled within 18 transformed sites (representing agricultural and urban land-uses) and neighbouring untransformed grassland.
RESULTS: Endemic and threatened species were negatively affected by transformation, particularly species with belowground bud-banks and storage organs. Species composition, with clear shifts in dominant families, was changed by over 90% on average by transformation
CONCLUSION: Land-use transformation lead to the loss of native species and increased alien invasive species.
Introduction
Land-use change threatens the persistence of many grassland ecosystems worldwide (Bond 2016). Grasslands are hyper-diverse ancient ecosystems, habitats and communities, supporting many endemic and threatened species (Carbutt, Henwood & Gilfedder 2017). Habitat transformation threatens the integrity of these systems through soil disturbance and the removal of plant biomass and species, and the effect is widely recognised and measurable (Herben, Chytry & Klimesová 2016; Miller, Roxburgh & Shea 2011). The poor understanding of forb dynamics in grassland necessitates a closer look at flo-ristic change and whether land-use change leads to species losses or gains in transformed grassland (Veldman et al. 2015).
In South Africa the Grassland Biome covers approximately one third of the land surface (Carbutt et al. 2011). The extent of grassland is defined on the basis of vegetation structure, as well as environmental factors including mean summer rainfall and minimum winter temperatures (Mucina & Rutherford 2006). The Grassland Biome is one of the most at-risk South African biomes, with 40-60% irreversibly modified, and less than 3% formally protected (Little, Hockey & Jansen 2015). The intactness of unprotected South African grasslands is threatened as there is an increase in the intensity of agriculture and afforestation (O'Connor & Kuyler 2009; Botha et al. 2017) and urban and industrial development activities (Siebert, Van Wyk & Bredenkamp 2001; O'Connor & Kuyler 2009). Changes in composition, structure and functioning of these grasslands influence the ability to deliver fresh water, soil formation, climate regulation and reduction of disaster risk (Egoh et al. 2011), and in addition, probable loss of biodiversity and grassland production (Everson & Everson 2016).
O'Connor and Kuyler (2009) have meticulously investigated the impact of land-use on the biodiversity integrity of moist grasslands in South Africa and highlighted the loss of useful plants from an ecosystem services perspective. Our study focusses on biodiversity intact-ness in that it specifically considers loss of native floristic diversity. It places special emphasis on the indigenous forb component that is fast moving up the research agenda (Siebert & Dreber 2019).
Materials and methods
Study area
Eighteen study sites were selected in four bioregions of the Grassland Biome, as well as a tropical bioregion of the Indian Ocean Coastal Belt Biome of South Africa (Figure 1). The chosen grasslands occurred at altitudes ranging between 30 and 3 100 m above sea level, with ten sites between 1 000 and 1 800 m. The mean annual temperature for the grassland sites ranged from 10 to 21°C, with an overall mean of 16.3°C (median 15.9°C). June to August are the coldest months with mean frost days per annum varying between 0 and 96, with a mean of 25 (median 28) across all study sites (Mucina & Rutherford 2006). All sites experience summer rainfall ranging from 600 to 1 000 mm per year and a mean of 761 mm (median 717 mm) across sites. Twelve sites receive less than 800 mm per annum.
Field surveys
Two dominant land transformation types in the Grassland Biome were included in this study, namely agriculture and urbanisation (Neke & Du Plessis 2004). Floristic data were gathered from 18 sites. At each site, sampling was conducted in four plots in untransformed grassland, each paired with a plot in an adjacent transformed land-use (i.e. eight plots per site), no more than 150-250 m apart. All 144 plots were surveyed in late spring or early to mid-summer. Each 100 m2 plot was divided into 25 subplots of 4 m2 each to record species occurrence and abundance. Species were identified in the field and photos were taken for later confirmation. Floristic data from the subplots were combined to compile a total inventory for each 100 m2 plot.
Plant species nomenclature and classification follow Ranwashe (2019). Naturalised and invasive categories are according to Department of Environmental Affairs (2016). Life and growth forms of plant species were obtained from Germishuizen and Meyer (2003). Categories of threat were obtained from the Red Data List of South African Plants (South African National Biodiversity Institute 2017).
Species abundance (N) was calculated as the total number of individuals and species richness (S) as the total number of species within each 100 m2 plot. The Shannon-Wiener (H') and Pielou (J') indices were applied to plot data to calculate alpha diversity and evenness respectively. All above values were calculated using Primer (2007).
Data analysis
Non-Metric Multi-Dimensional Scaling (NMDS) analysis in Primer (2007) was used to explore changes in species composition between transformed and un-transformed grasslands. Permutational Multivariate Analysis of Variance (PERMANOVA) was performed using species abundance data. Analyses were conducted with 999 permutations using Bray-Curtis similarity and Type III sums of squares after a square root transformation of species data to reduce the influence of common species. To account for location variability in the paired, nested sampling design, plots were treated as a random variable nested within a transformation type (i.e. urban or agricultural), which were treated as the fixed factor. Pair-wise test results indicated the strength of the difference between transformed and untransformed plots.
Similarity Percentage Analysis (SIMPER) was applied to determine which forb and grass species contributed the most to differences between transformed and obtained from Germishuizen and Meyer (2003). Categories of threat were obtained from the Red Data List of South African Plants (South African National Biodiversity Institute 2017).
Results
Floristics
Overall, 1 146 plant species were recorded, of which 144 were non-native. The untransformed grassland contained 962 species, which included 35 naturalised and 15 invasive taxa (5%), 175 South African endemics (18%) and 20 threatened species (2%). The transformed grasslands had 582 species, including 92 naturalised and 46 invasive taxa (24%), 47 South African endemics (8%) and six threatened species (1%).
The most prominent families in the localities were the Asteraceae, Poaceae, Fabaceae and Cyperaceae in order of most species diverse (Table 1), whereas the Poa-ceae were most abundant (Table 2). It is evident that transformation is less favourable to the Asteraceae and Fabaceae, and more beneficial to the Cyperaceae and especially the Poaceae.
Habitat transformation affects the number of species present per family. The geophytic Hyacinthaceae and Iridaceae showed the largest species losses (18 and 19 species respectively) when grassland is transformed and the weedy Amaranthaceae and Solanaceae benefitted in terms of species additions (9 and 5 species respectively; Table 1). Changes in the frequency of species is even more pronounced (Table 2). Five of the top ten families that have high frequencies of occurrence in un-transformed grassland become reduced in transformed grassland by 73%. These are replaced by five families, which in turn showed a 75% increase in transformed grassland (Table 2).
Composition
Changes in the species number and frequency of families is expected to have an effect on the composition of transformed grassland. The results from a NMDS revealed clustering that supports the untransformed and transformed grasslands as separate assemblages (Figure 2). Results from the pair-wise tests in PERMANOVA indicated a significant difference in floristic composition between transformed and untransformed grasslands in both urban (df = 70, t = 2.17, p = 0.001) and agricultural (df = 70, t = 2.88, p = 0.001) transformation types (Figure 2). Bray Curtis similarity measures in the PERMANOVA design reported a low 6.96% and 5.7% similarity in species composition between transformed and untransformed agricultural and urban grasslands respectively. This implies that transformation changed species composition in grasslands by ~90% on average. Fifteen most common grass species explained 21.26% of the dissimilarity between transformed and untrans-formed grasslands, with species such as Cynodon dacty-lon and Hyparrhenia hirta weighted towards the former and Digitaria eriantha and Themeda triandra towards the latter (Table 3). Comparatively the first 15 forbs species only contributed 8.52% to the dissimilarity, with species such as Cyperus esculentus and Richardia brasil-iensis weighted towards transformed, and Helichrysum rugulosum and Scabiosa columbaria towards untrans-formed grassland (Table 3).
Diversity
Changes in the species composition are expected to have an effect on species richness and diversity in transformed grassland. Simple paired t-tests revealed significantly lower diversity (for all measures, i.e. J', H', S and N) in the transformed grassland (p<0.001, Table 4). Species richness decreased by nearly 50%.
Status
The lower evenness in transformed grassland indicates uneven proportional contribution of individuals between species and is indicative of some species becoming more dominant and others becoming marginal. Dominance shifts can be ascribed to increased numbers of alien, invasive and annual species in the transformed grassland, with the former two being >80% lower in untransformed grassland (Table 4). Threatened and endemic species richness and abundance decreased by >80% in the transformed grassland (Table 4).
Growth forms
When aliens displace extant species (including endemics), certain growth forms become more or less prominent. Species losses (Table 4) were recorded for species with underground storage organs and bud-banks (USOs), such as geophytes (>80%), parasitic plants and suffrutices (>90%). Succulents were considered separately from the dwarf shrub growth form and were also decreased by over 90% in transformed grasslands (Table 4).
Discussion
Asteraceae and Fabaceae were the most dominant forb families in both transformed and untransformed grassland, a trend that has previously been reported (Botha et al. 2017). It can be deduced that regenerative traits, such as wind dispersal and seed dormancy for rapid colonisation (Asteraceae), ballochory and/or or endozo-ochory as seed dispersal traits, and resource acquisition traits, which increase resprouting capacity (Fabaceae), make them rather resilient to disturbance. However, the Amaranthaceae, Brassicaceae, Solanaceae and
Verbenaceae benefit from the transformation of grasslands and their numbers and dominance increase through the introduction of weedy, mostly alien, species. These groups are renowned for their ability to colonise frequently transformed man-made habitats (Pysek, Prach & Smilauer 1995). In contrast, certain families, such as the geophytic Hyacinthaceae and Iridaceae, were extensively disadvantaged by habitat transformation, as they are sensitive to soil disturbance because their bud-banks are belowground (Fidelis et al. 2014). The general trend is therefore one of species loss and displacement by a new flora, mostly annuals, with colonising traits better suited to a transformed environment, such as creepers, clonal plants and fruit or seed adapted for exozoochorous or anemochorous dispersal (Botha et al. 2017).
Many species of ancient grasslands are not tolerant to anthropogenic disturbance (Siebert 2011). Common native species disappeared completely where grasslands were transformed, such as the grasses Alloterop-sis semialata (R.Br.) Hitchc. and Schizachyrium san-guineum (Retz.) Alston, forbs Gerbera ambigua (Cass.) Sch.Bip. and Haplocarpha scaposa Harv., geophytes Ledebouria luteola Jessop and Hypoxis argentea Harv. ex Baker., dwarf shrubs Athrixia phylicoides DC. and Tephrosia capensis (Jacq.) Pers., and suffrutices Ele-phantorrhiza elephantina (Burch.) Skeels and Ziziphus zeyheriana Sond. Other species, such as the palatable and productive grass, Themeda triandra, which is considered a keystone species and indicator of undisturbed grassland (Snyman, Ingram & Kirkman 2013), were severely reduced. Many new, mostly alien species, enter the transformed system, providing species that can be considered indicators of disturbance (Morris & Scott-Shaw 2019), such as the prostrate and grazing-resistant, Richardia brasiliensis. This study confirmed the results of O'Connor (2015) that a substantial decrease in T. triandra and increase in R. brasiliensis are indicative of transformed grassland.
As previously shown, untransformed grasslands have greater plant species diversity (27%) and richness (92%) than transformed grasslands (O'Connor 2005; Siebert 2011). Species loss was specific to certain growth forms with underground organs specifically adapted to survive harsh winter conditions, drought and fire (Bond & Parr 2014; Bond 2016). The loss of these and other foundation species open up niches for colonisation by alien species (Prevéy et al. 2010). This is problematic, as loss of native species hampers the grassland ecosystem from fulfilling all its functions (Zavaleta et al. 2010). Overall, the situation in transformed grassland is not only one of species depletion, but increases in woody growth form dominance is predicted to become more pronounced in South African grasslands by 2030 (Gibson et al. 2018).
Conclusion
Major plant families remain floristically dominant after transformation, but there is a negative impact on overall phylogenetic diversity and the promotion of the Poa-ceae and Cyperaceae. Non-typical grassland families, with a wide array of disturbance-tolerant traits, show an increase in phylogenetic diversity, which is mainly a consequence of the introduction of alien weedy species.
Species composition of grassland was transformed by disturbance and is indicative of better adapted species entering the system or existing pre-adapted ones becoming more dominant due to competition release and/or altered microclimates and soils (Pysek, Prach & Smilauer 1995). This is evidenced by the proportion of grass species increasing, but a large reduction in forb species with USOs (Fidelis et al. 2014). No evidence was found for extensive woody encroachment in transformed areas.
This study set out to assess species loss due to transformation and, based on the current data set, it can be conclusively stated that grassland is severely impacted in terms of its species richness and diversity. These changes are of concern as grasslands have high economic value and support the wellbeing of humans by providing, among others, ecological infrastructure, carbon sinks, albedo surfaces, plant-based medicines, food plants and grazing for livestock (Bengtsson et al. 2019). Further studies are needed to determine whether these floristic shifts can still maintain and provide the ecosystem services that are expected from grasslands in South Africa.
Acknowledgements
Our appreciation to Dr Monique Botha, Dr Elandrie Davoren and Mr Paul Janse van Rensburg for making plot data available for this study, and to Mr Wynand Muller for producing the locality map. The South African National Biodiversity Institute, South African Environmental Observation Network, National Research Foundation of South Africa and Letseng Diamond Mine, Lesotho, provided financial support to the researchers and students involved in this project.
Authors' contributions
MM collected field data, conducted data analyses and contributed to the writing of the manuscript. SJS planned and coordinated the study, collected field data, conducted data analyses and co-wrote the manuscript. BRN planned and coordinated part of the study, collected field data, and conducted data analyses. FS collected field data and contributed to the writing of the manuscript.
Disclaimer
The views expressed in the submitted article are our own and not an official position of the institution or funder.
Source(s) of support
South African National Biodiversity Institute, South African Environmental Observation Network, National Research Foundation.
References
Bengtsson, J., Bullock, J.M., Egoh, B., Everson, C., Everson, T., O'Connor, T., O'Farrell, PJ., Smith, H.G. & Lindborg, R., 2019, 'Grasslands - more important for ecosystem services than you might think', Ecosphere 10(2), p.e02582. https://doi.org/10.1002/ecs2.2582 [ Links ]
Bond, W.J., 2016, 'Ancient grasslands at risk', Science 351 (6269),120-122. https://doi.org/10.1126/science.aad5132 [ Links ]
Bond, W.J., & Parr, C.L., 2010, 'Beyond the forest edge: ecology, diversity and conservation of the grassy biomes', Biological Conservation 143(10), 2395-2404. https://doi.org/10.1016/j.biocon.2009.12.012 [ Links ]
Botha, M., Siebert, S.J., Van den Berg, J., Ellis, S. & Dre-ber, N., 2017, 'Plant functional types differ between the grassland and savanna biomes along an agro-ecosystem disturbance gradient in South Africa', South African Journal of Botany 113, 308-317. https://doi.org/10.1016/j.sajb.2017.09.008 [ Links ]
Carbutt, C., Henwood, W.D. & Gilfedder, L.A., 2017, 'Global plight of native temperate grasslands: going, going, gone?', Biodiversity and Conservation 26(12), 2911-2932. https://doi.org/10.1007/s10531-017-1398-5 [ Links ]
Carbutt, C., Tau, M., Stephens, A. & Escott, B., 2011, 'The conservation status of temperate grasslands in southern Africa', The Grassland Society of Southern Africa 11(1), 1723. https://grassland.org.za/publications/grassroots/issues/february-2011/5%20201102%20Carbutt.pdf [ Links ]
Department of Environmental Affairs, 2016, National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004) Alien and invasive species lists, Government Gazette 40166(864), Pretoria, pp. 31-104. [ Links ]
Everson, C.S. & Everson, T., 2016, 'The long-term effects of fire regime on primary production of montane grasslands in South Africa', African Journal of Range & Forage Science 33(1), 33-41. https://doi.org/10.2989/10220119.2015.1124922 [ Links ]
Fidelis, A., Appezzato-da-Glória, B., Pillar, V.D. & Pfadenhauer, J., 2014, 'Does disturbance affect bud bank size and be-lowground structures diversity in Brazilian subtropical grasslands?', Flora 209(2), 110-116. https://doi.Org/10.1016/j.flora.2013.12.003 [ Links ]
Germishuizen, G. & Meyer, N.L., 2003, Plants of southern Africa: an annotated checklist, National Botanical Institute, Pretoria. [ Links ]
Gibson, L., Münch, Z., Palmer, A. & Mantel, S., 2018, 'Future land cover change scenarios in South African grasslands - implications of altered biophysical drivers on land management', Heliyon 4(7), e00693. https://doi.org/10.1016/j.heliyon.2018.e00693 [ Links ]
Herben, T., Chytry, M. & Klimesová, J., 2016, 'A quest for species level indicator values for disturbance', Journal of Vegetation Science 27(3), 628-636. https://doi.org/10.1111/jvs.12384 [ Links ]
Little, I.T., Hockey, PA.R. & Jansen, R., 2015, 'Impacts of fire and grazing management on South Africa's moist highland grasslands: A case study of the Steenkampsberg Plateau, Mpumalanga, South Africa', Bothalia 45(1), Art. #1786. https://dx.doi.org/10.4102/abc.v45i1.178 [ Links ]
Miller, A.D., Roxburgh, S.H. & Shea, K., 2011, 'How frequency and intensity shape diversity-disturbance relationships', Proceedings of the National Academy of Sciences 108(14), 5643-5648. https://doi.org/10.1073/pnas.1018594108 [ Links ]
Morris, C.D. & Scott-Shaw, R., 2019, 'Potential grazing indicator forbs for two mesic grasslands in South Africa', Ecological Indicators, 107, 105611. https://doi.org/10.1016/j.ecolind.2019.105611 [ Links ]
Mucina, L. & Rutherford, M.C., 2006, The vegetation of South Africa, Lesotho and Swaziland, South African National Biodiversity Institute, Pretoria. [ Links ]
Neke, K.S. & du Plessis, M.A., 2004, 'The threat of transformation: quantifying the vulnerability of grasslands in South Africa', Conservation Biology 18, 466-477. https://doi.org/10.1111/j.1523-1739.2004.00157.x [ Links ]
O'Connor, T.G., 2005, 'Influence of land use on plant community composition and diversity in Highland Sourveld grassland in the southern Drakensberg, South Africa', Journal of Applied Ecology 42(5), 975-988. https://doi.org/10.111/j.1365-2664.2005.01065.x [ Links ]
O'Connor, T.G. & Kuyler, P., 2009, 'Impact of land use on the biodiversity integrity of the moist sub-biome of the grassland biome, South Africa', Journal of Environmental Management 90(1), 384-395. https://doi.org/10.1016/j.jenvman.2007.10.012 [ Links ]
Prevéy, J.S., Germino, M.J., Huntly, N.J. & Inouye, R.S., 2010, 'Exotic plants increase and native plants decrease with loss of foundation species in sagebrush steppe', Plant Ecology 207(1), 39-51. https://doi.org/10.1007/s11258-009-9652-x [ Links ]
Primer, 2007, Primer, Version 6, Primer-E Ltd, United Kingdom. [ Links ]
Pysek, P., Prach, K. & Smilauer, P., 1995, 'Relating invasion success to plant traits: an analysis of the Czech alien flora', in P Pysek, K. Prach, M. Rejmánek & M. Wade (eds), Plant invasions: General aspects and special problems, pp. 39-60, Academic Publishing, Amsterdam. [ Links ]
Ranwashe, F., 2019, BODATSA: Botanical Collections, v1.4, South African National Biodiversity Institute, viewed 15 May 2019, from http://ipt.sanbi.org.za/iptsanbi/re-source?r=brahms_online&v=1.4 [ Links ]
Siebert, F. & Dreber, N., 2019, 'Forb ecology research in dry African savannas: Knowledge, gaps, and future perspectives', Ecology & Evolution 9(13), 7375-7891. https://doi.org/10.1002/ece3.5307 [ Links ]
Siebert, S.J., 2011, 'Patterns of plant species richness of temperate and tropical grassland in South Africa', Plant Ecology and Evolution 144(3), 249-254. https://doi.org/10.5091/plecevo.2011.501 [ Links ]
Siebert, S.J., Van Wyk, A.E. & Bredenkamp, G.J., 2001, 'Ende-mism in the flora of ultramafic areas of Sekhukhuneland, South Africa', South African Journal of Science 97(11): 529-532. https://journals.co.za/content/sajsci/97/11-12/EJC97254 [ Links ]
Snyman, H.A., Ingram, L.J. & Kirkman, K.P, 2013, 'Themeda triandra: a keystone grass species', African Journal of Range & Forage Science 30(3), 99-125. https://doi.org/10.2989/10220119.2013.831375 [ Links ]
South African National Biodiversity Institute, 2017, Red List of South African Plants version 2017.1, viewed 1 June 2019, from http://redlist.sanbi.org/ [ Links ]
Statistica, 2017, Statistica, version 13, TIBCO Software Inc., United States of America. [ Links ]
Veldman, J.W., Buisson, E., Durigan, G., Fernandes, G.W., Le Stradic, S., Mahy, G., Negreiros, D., Overbeck, G.E., Veldman, R.G., Zaloumis, N.P. & Putz, F.E., 2015, 'Toward an old growth concept for grasslands, savannas, and woodlands', Frontiers in Ecology and the Environment 13(3), 154-162. https://doi.org/10.1890/140270 [ Links ]
Zavaleta, E.S., Pasari, J.R., Hulvey, K.B. & Tilman, G.D., 2010, 'Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity', Proceedings of the National Academy of Sciences 107(4), 1443-1446. https://doi.org/10.1073/pnas.0906829107 [ Links ]
 Correspondence:
Correspondence:
Prof. S.J. Siebert,
stefan.siebert@nwu.ac.za
Submitted: 20 September 2019
Accepted: 1 December 2020
Published: 24 February 2021
SHORT COMMUNICATION
A baseline assessment of the photosynthetic potential of Welwitschia mirabilis using the JIP-test for monitoring and conservation purposes
J.M. BernerI; B H. CloeteI; T. ShuuyaII
IUnit for Environmental Sciences and Management, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa
IIGobabeb Research and Training Centre, Namib Naukluft Park, Namibia, P.O. Box 953, Walvisbay 13103, Namibia
ABSTRACT
BACKGROUND: Welwitschia mirabilis is highly specialised to survive the harsh climate of the Namib Desert. Changes in land use, such as the expansion of mining activities, may endanger their survival.
OBJECTIVES: The purpose of this study was to understand the photosynthetic potential of W. mirabilis plants to provide a baseline for future long-term monitoring, and for future comparison to determine plant health status after the onset of mining operations
METHODS: The study was conducted in a population of W. mirabilis on the Welwits-chia Plains. Chlorophyll a fluorescence data were used to measure plant photochemical potential and analysed using the JIP-test.
RESULTS: Significant differences in the photosynthetic potential was observed for W. mirabilis plants located in different catchments. The partial parameters of the PIABS values were also significantly lower, which indicated that all aspects of photosynthesis were influenced
CONCLUSION: PIABS values can serve as a baseline for future long-term monitoring studies to detect any changes in the health status of W. mirabilis that might result from land use change.
Keywords: chlorophyll a fluorescence, JIP-test, photosynthesis, PIABS, Welwitschia mirabilis, Welwitschia Plains
Introduction
Welwitschia mirabilis Hook.f. (Welwitschiaceae) is undoubtedly a desert oddity and, unlike most desert plants, has relatively large leaves. Its sheer size in comparison with other desert xerophytes emphasises its uniqueness (Veste 2008), together with its anatomy, cytology and habitat in which it is found (Schulze et al. 1976). W. mirabilis also has an unusual metabolic pathway; even though it displays a C3 photosynthetic pathway, it also exhibits CAM characteristics (Cooper-Driver 1994; Henschel & Seely 2000; von Willert et al. 2005).
Though more than one population of W. mirabilis exists within the Namib Desert, the Welwitschia Plains have the most plants and these are also the best-studied specimens (World Heritage Convention 2002). This area lies within the Namib-Naukluft Park and is the most accessible location for tourists to see these remnants from the Jurassic period (Cooper-Driver 1994). W. mirabilis plants form the dominant perennial vegetation in the area and provide shelter for numerous desert creatures such as arachnids, lizards and birds, and sustenance for oryx and zebra.
Mining in Namibia contributes substantially to its economy (Humavindu 2013), and uranium is currently being mined close to the Welwitschia Plains. This change in land use may pose a threat to the health and integrity of the surrounding desert ecosystem and, therefore, to the protection of W. mirabilis plants. The development of a management and monitoring plan to ensure the future of these iconic plants would be imperative, since W mirabilis is a protected species under the Namibian Forest Act, No. 12 of 2001 and is also listed in Appendix II of the Convention on International Trade in Endangered Species (CITES).
Considering the species' protection status, a nondestructive and cost-effective method is required for monitoring. Chlorophyll a fluorescence-based techniques to assess plant health status, such as the JIP-test, is non-intrusive and widely employed to monitor stress (Busotti et al. 2010). Plants emit a fluorescence signal at a wavelength higher than 690 nm after exposure to actinic light. The JIP-test is then used to analyse the polyphasic rise of the chlorophyll fluorescence signal to gather valuable information about the plant's photosynthetic system (Strasser et al. 2004). Changes in the chemical and physical environment will lead to changes in the shape of the fluorescence transient and, therefore, it can be used to investigate the photosyn-thetic potential of plants. This study's objective was to understand the photosynthetic potential of W. mirabilis plants on the Welwitschia Plains to provide a baseline for future long-term monitoring of the plant health status for conservation purposes after the onset of mining operations.
Materials and method
The Welwitschia Plains are located approximately 60 km east of Swakopmund in the central Namib Desert, enclaved between the Swakop and Khan rivers (World Heritage Convention 2002). This area is characterised by rocky outcrops, inselbergs, rocky valleys, drainage networks and plains.
Rainfall patterns in the central Namib Desert are sporadic with an increase from the coast (~10 mm) eastwards (~60 mm at 100 km inland) (Shanyengana et al. 2002). Fog and dew are the primary water sources for many plants in the central Namib (Henschel & Seely 2000). Unlike rainfall, fog events and amount decrease with the distance from the coast, with 60-200 days of fog events recorded at Gobabeb (Henschel & Seely 2000; Shanyengana et al. 2002).
The study area was subdivided into three catchments areas due to observed differences in geological formations and topography, namely Campsite, River Channel and Zone 6 (Figure 1). Within each of these catchments, 12 individual plants were randomly selected for monitoring using ArcGIS 10.2 software (Supplementary Table 1) and were located in the field with their respective GPS coordinates.
Chlorophyll a fluorescence measurements of W. mirabi-lis plants were taken during winter (July) with a Handy Pea (Plant Efficiency Analyzer) fluorometer during the night. Measurements were taken one hour after sunset and continued to approximately midnight. Such dark adaption was done to ensure that all reaction centres were open. Ten fluorescence measurements were taken at different spots on each plant within the first 10 cm from the leaf base. During the time of the measurements, the night temperature was around 18°C. The Handy Pea was calibrated to produce pulses of light with an intensity of 3 445 ^mol mol-1 and a gain of 1. These light pulses each had a duration of 1 second at a wavelength of 650 nm.
The chlorophyll fluorescence induction curve has specific inflection points named O, K, J, I and P plotted on a logarithmic time scale (OJIP transient). Typically, stress will influence the shape of the OJIP transients by causing a shift in the induction curve. Assessing shifts in the induction curve provides information regarding the photosynthetic potential of the plant and, ultimately, plant health. The OJIP transient section between steps J and P is known as the thermal phase (or the multiple turn-over phase). This phase represents the reduction of the electron transport chain. The step between J and I is associated with the reduction of the PQ-pool and the I to P step with electron flow through photosystem I (Stirbet & Govindjee 2011).
The performance index (PIABS) is a widely used JIP-test parameter that provides quantitative information about the physiological state of plants and vitality. PIABS provides information about the potential for energy conservation from light absorption to the reduction of intersystem electron acceptors (Strasser et al. 2004). It is a function of its three partial parameters: the density of active reaction centres per chlorophyll (yRC/(1-yRC)), the efficiency of electron movement by trapped excitation into electron transport chain ((ppO/(1-(ppO)) and the probability that the reaction centres will trap an absorbed photon (i|/Eo/(1-i|/Eo)) (Strasser et al. 2000). If a stress condition influences any of these partial parameters, the stress will influence the PIABS values and reflect the current state of photosyn-thetic performance (Strasser et al. 2004).
The fluorescence data captured with the Handy Pea was analysed with PEA Plus 1.140 software. The River Channel was selected as the reference site as the plants represented at this site were visually larger and appeared healthier compared to the other two sites. The translation of the fluorescence data to biophysical parameters was done according to Strasser et al. (2004). Data were subjected to the Shapiro-Wilk test and if data conformed to the assumptions of normality, a one-way ANOVA was run for each parameter in Statistica v13, Dell Inc. (2016).
Results
Typical chlorophyll a fluorescence induction curves of dark-adapted leaves of W. mirabilis plants were plotted for the Campsite, River Channel and Zone 6 catchments (Figure 2). The time frame between steps O and J (also referred to as the single turn-over phase or the photochemical phase) provides information about the antenna size and the connectivity between photosystem II reaction centres (Strasser et al. 2004). When the induction curves from the different catchments were compared to one another during this time frame, there were no apparent shifts in the induction curve from any of the catchments (Figure 2). A shift in the shape of the Campsite induction curve was observed between steps I and P. The rise in the fluorescence transient, especially after the I-step, was the lowest at Campsite. This would imply that the electron flow between photosystem II and photosystem I was less efficient (Figure 2).
W. mirabilis plants located at Campsite had significantly lower PIABS (P < 0.05) values when compared to the other catchments (Figure 3). The lower PIABS values of Campsite suggests that these W. mirabilis plants are in a less optimal condition. By normalising the JIP parameters of Campsite and Zone 6 to River Channel (references site), differences among the catchments were emphasised. From the spider plot it was clear that all of the parameters that comprise the PIABS values for Campsite were all lower than those of River Channel and Zone 6 (Figure 4). The density of active reaction centres (yRC/(1- YRC)), the potential to create a charge separation (φρ(_/(1- φ )) and the potential to transport electrons between pSiI and PSI (v|/Eo/(1-v|/Eo)) were all lower at Campsite (Figure 4).
The total performance index, PItotal, is the product of the PIABS and the probability that an electron can move from a reduced intersystem electron acceptor to the PSI end-electron acceptors (Tsimilli-Michael & Strasser 2008). The PI index is, therefore, very closely related to the plants' overall growth and health. The PI values of the welwitschia plants growing at Campsite were also significantly lower (P < 0.05). The only difference between the PItotal and the PIabs is that for calculating the PItotal index, the reduction of the end electron acceptors (5Ro/(1-5Ro)) is included. Overall the spider plot (Figure 4) clearly shows that the photosynthetic potential of the W. mirabilis plants at Campsite was less efficient compared to the other two catchments.
Discussion
Monitoring the health status of W. mirabilis plants is critical for early detection of the impact of land-use change. Chlorophyll a fluorescence measurements, which were taken before the onset of mining activi-tiesm, can now serve as a baseline for future monitoring
(Kalaji et al. 2016). The vitality statuses of plants before mining activities is indicated by differences in the photosynthetic potential between W. mirabilis plants located within different catchment areas in the same locality. The maximum photosynthetic quantum yield of W. mirabilis plants located at Campsite was lower than River Channel and Zone 6, indicating a less optimal health condition when compared to the latter two.
The Campsite catchment has a higher elevation than the other catchments resulting in lower water accumulation, as water accumulates along the flow paths, which is influenced by the topography (Fan et al. 2020). This lower water accumulation at Campsite will lower the photosynthetic potential of the W. mirabilis plants. Topographic features such as elevation and slope may also change vegetation exposure to wind and solar radiation, contributing to a decrease in the photosynthetic potential (Mikita & Klimánek 2010). Because this study was conducted during winter, episodic rainfall did not influence the measurements. Incoming fog from the Atlantic Ocean might influence the photosynthetic potential of plants and has to be acknowledged, but the selected welwitschia plants for this study were chosen from outside the reach of the incoming fog.
For all practical reasons, the PItotal performance index or any partial parameters could have been used (Kalaji et al. 2016). To optimise the value of the JIP-test, annual readings should be taken on the same plants and any changes in the JIP parameters should be noted. It is recommended that several measurements be taken throughout the year. The data from this study represent the environmental conditions during the winter and if this same investigation was carried out during the summer, different PIABS values would be obtained, but the trend should remain the same (Janssen & Hasselt 1994).
Considering the planned change in anthropogenic activities, together with natural stressors on the fringes of the Welwitschia Plains, it is imperative to detect changes in the health status before the onset of visible stress symptoms. This early detection of plant stress will prompt for management actions to prevent populations from being adversely affected (Chaerle & Van Der Straeten 2000). Therefore, the chlorophyll fluorescence parameters analysis can be a very informative tool in ecological surveys (Kalaji et al. 2016) by providing explanations on the physiological behaviour of W. mirabilis plants in response to its changing environment. We suggest that long-term monitoring studies integrating potential drivers and responses be conducted to understand the plant health of W. mirabilis across the landscape. This study has established a baseline that can be used to develop a protocol to monitor the plant physiological status and the possible management strategies for mines and other developments that may have adverse impacts on the W. mirabilis population. Besides that, the findings may also aid restoration and rehabilitation measures such as transplantation and re-introduction of this species by understanding its current functional health status across the landscape over time.
Conclusion
Chlorophyll a fluorescence measuring techniques have high potential to investigate plant health in situ in longterm monitoring. Our study was a preliminary one, conducted over only a short period. Considering the longevity of welwitschia plants and the urgent need to develop a thorough understanding of how the species reacts to different stressors created by land-use change, longer-term studies should be conducted to understand the in situ spatial and temporal patterns of the species' health. With increasing mining activities on the fringes of the Welwitschia Plains and the potential threat that these anthropogenic activities pose to the welwitschia population, continued monitoring is vital.
Acknowledgments
The authors of this paper would like to thank the National Research Foundation (NRF) in South Africa and the National Commission on Research, Science and Technology (NCRST) of Namibia for funding this project. Also, our appreciation to the Gobabeb Research and Training Centre, Namibia, for organising the permits to conduct research within the Welwitsch-ia Plains and to the Gobabeb volunteers who assisted with the collection of data.
Authors' contributions
JMB planned and coordinated the study, collected field data, conducted data analyses and wrote the manuscript. HC collected field data, conducted data analyses and contributed to the writing of the manuscript. TS collected field data and contributed to the analysis & interpretation of the data.
Disclaimer
The views expressed in the submitted article are our own and not an official position of the institution or funder.
References
Busotti, F., Desotgiu, R., Pollastrini, M. & Cascio, C., 2010, 'The JIP test: a tool to screen the capacity of plant adaptation to climate change', Scandanavian Journal of Forest Research, 25(8), 43-50, https://doi.org/10.1080/02827581.2010.485777. [ Links ]
Chaerle, L. & Van Der Straeten, D., 2000, 'Imaging techniques and the early detection of plant stress', Trends in Plant Science, 5(11), 495-501, https://doi.org/10.1016/S1360-1385(00)01781-7. [ Links ]
Cooper-Driver, G., 1994, 'Welwitschia mirabilis - a dream come true', Arnoldia, 54(2), 2-10. [ Links ]
Henschel, J. & Seely, M.K., 2000, 'Long-term growth patterns of Welwitschia mirabilis, as long-lived plant of the Namib Desert', Plant Ecology, 150(1-2), 7-26, https://doi.org/10.1023/A:1026512608982. [ Links ]
Fan, J., Xu, Y., Ge, H. & Yang, W., 2020, 'Vegetation growth variation in relation to topography in Horqin Sandy Land', Ecological Indicators, 113, 106-215. [ Links ]
Humavindu, M. & Stage, J., 2013, 'Key sectors of the Namib-ian economy', Journal of Economic Structures, 2(1), 1-15, https://doi.org/10.1186/2193-2409-2-1. [ Links ]
Janssen, L.H. & Hasselt, PR., 1994, 'Temperature effects on chlorophyll fluorescence induction in tomato', Journal of Plant Physiology, 144(2), 129-135. [ Links ]
Kalaji, H.M., Jajoo, A., Oukarroum, A., Brestic, M., Zivcak, M., Samborska, I.A. & Ladle, R.J., 2016, 'Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions', Acta Physiologiae Plantarum, 38(4), 102, https://doi.org/10.1007/s11738-016-2113-y. [ Links ]
Mikita, T. & Klimánek, M., 2010, 'Topographic exposure and its practical applications', Jounal of Landscape Ecology, 3(1), 42-51. [ Links ]
Schulze, E.D., Ziegler, H. & Stichler, W., 1976, 'Environmental control of Crassulacean Acid Metabolism in Welwitsch-ia mirabilis Hook. Fil. in in its range of natural distribution in the Namib desert', Oecologia, 24, 323-334. [ Links ]
Shanyengana, E.S., Henschel, J.R., Seely, M.K. & Sanderson, R.D., 2002, 'Exploring fog as a supplementary water source in Namibia', Atmospheric Research, 64(1), 251-259, DOI: 10.1016/S0169-8095(02)00096-0. [ Links ]
Stirbet, A., 2011, 'On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basic applications of the OJIP fluorescent transient', Journal of Photochemistry & Photobiology B: Biology, 104, 236-257, DOI: 10.1016/j.jphotobiol.2010.12.010. [ Links ]
Strasser, R.J., Srivastava, A. & Tsimilli-Michael, M., 2000, 'The fluorescence transient as a tool to characterize and screen photosynthetic samples', in M. Yunus, U. Pathre & P. Mohanty (eds), Probing photosynthesis: Mechanisms, regulation and adaptation, London: Taylor & Francis, pp. 445-483. [ Links ]
Strasser, R.J., Tsimilli-Michael, M. & Srivastava, A., 2004, 'Analysis of the chlorophyll a Fluorescence Transient', in G.C. Papageorgiou & Govindjee (eds), Chlorophyll a fluorescence. A signature of photosynthesis. Advances in Photosynthesis and Respiration, vol 19. Dordrecht: Springer, p. 818. [ Links ]
Tsimilli-Michael, M. & Strasser, R.J., 2008, 'In vivo assessment of stress impact on plant's vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants', in A. Varma (ed.), Mycorrhiza. Berlin: Springer. [ Links ]
Veste, M. & Herppich, W.B., 2008, 'Welwitschia mirabilis -Eine ökophysiologische Betrachtung', Naturwissenschaftliche Rundschau, 61, 620-624. [ Links ]
von Willert, D.J., Armbrüster, N., Drees, T. & Zaborowski, M., 2005, 'Welwitschia mirabilis: CAM or not CAM - what is the answer?', Functional Plant Biology, 32(5), 389-395, DOI: 10.1071/FP01241. [ Links ]
World Heritage Convention, 2002. Welwitschia plains. Available at: http://whc.unesco.org/en/tentativelists/1747/ (Accessed: 9 September 2015). [ Links ]
 Correspondence:
Correspondence:
Dr Jacques Berner
jacques.berner@nwu.ac.za
Submitted: 24 September 2019
Accepted: 15 October 2020
Published: 24 February 2021














