Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.49 n.1 Pretoria 2019
http://dx.doi.org/10.4102/abc.v49i1.2270
ORIGINAL RESEARCH
Vegetation, floristic composition and structure of a tropical montane forest in Cameroon
Moses N. SaingeI, II; Ngoh M. LyongaI; Gildas P.T. MbatchouIII; David KenfackIV; Felix NchuV; Andrew T. PetersonVI
ITropical Plant Exploration Group, Mundemba, Cameroon
IIDepartment of Environmental and Occupational Studies, Cape Peninsula University of Technology, South Africa
IIIProgramme for the Sustainable Management of Natural Resources, South-West Region, Cameroon
IVCenter for Tropical Forest Science, Smithsonian Institution, United States
VDepartment of Horticultural Sciences, Cape Peninsula University of Technology, South Africa
VIBiodiversity Institute, University of Kansas, United States
ABSTRACT
BACKGROUND: The Rumpi Hills Forest Reserve (RHFR) is a montane forest area in south-western Cameroon. Although RHFR is presumed to be rich in biodiversity and vegetation types, little information exists regarding its floristic composition and vegetation patterns.
OBJECTIVES: Our goal was to characterise vegetation patterns in the reserve and to understand how elevation influences distributions and diversity of species. We aimed to provide a first detailed plant species inventory for this important forest area, as well as basic information on forest structure.
METHOD: We characterised floristic composition and vegetation patterns of the reserve in 25 1-ha plots along an elevational gradient from 50 m to 1778 m. In each plot, trees and lianas of diameter at breast height (dbh) ≥ 10 cm were measured; shrubs < 10 cm were measured in nested plots of 0.01 ha.
RESULTS: In all, 16 761 trees, shrubs and lianas with dbh ≥ 1 cm were censused, representing 71 families, 279 genera and 617 morphospecies. Floristic composition ranged from 94 to 132 species, with a mean of 117.5 species per hectare in lowland forest (50 m - 200 m) and 36-41 species, with a mean of 38.5 species per hectare in montane cloud forest (1600 m - 1778 m) near the summit of Mount Rata. Two-way indicator species analysis classified the 25 plots into six vegetation types corresponding to lowland evergreen rainforest, lowland evergreen rainforest on basalt rocks, middle-elevation evergreen forest, submontane forest, transitional submontane forest and montane cloud forest. In all, 0.006% of the reserve was included in our sample plots. Detrended correspondence analysis highlighted the importance of elevation in shaping vegetation patterns.
CONCLUSION: The RHFR is composed of different vegetation types, which show impressive variation in terms of structure, species composition and diversity. The detailed, fine-scale inventory data obtained in this study could be useful in planning efficient management of this and other montane tropical forests.
Introduction
Floristic composition, forest structure and vegetation characterisation have been treated widely in tropical forest ecosystems (Campbell et al. 2006; Lutz et al. 2012; Neelo et al. 2015; Noumi 2013; Zhong et al. 2015), yet some tropical forest zones remain less studied. One such area is the tropical montane forest zone along the continental part of the Cameroon Volcanic Line (Ayonghe et al. 1999; Marzoli et al. 2000; Sainge et al. 2017), where few studies have assessed forest structure and composition along elevational gradients using permanent sampling plots (Gonmadje et al. 2011; Sainge 2016; Sunderland et al. 2003; Tchouto 2004). Rather, most work in this region has been based on more common general plant collection methods (e.g. Cheek, Onana & Pollard 2000; Thomas 1996, 1997).
Some studies have examined changes in species composition and diversity across environmental and geographic gradients (Gentry 1988; Imani et al. 2016), but vegetation structure and composition are also influenced strongly by elevation (Imani et al. 2016; Richter 2008). Vegetation systems at different elevations on different substrates in montane ecosystems differ in biomass production, carbon storage and biodiversity conservation value (Richter 2008). Globally, although the biodiversity of elevational gradients in the tropics have seen much attention (e.g. Desalegn & Beierkuhnlein 2003; Fischer, Blaschke & Bassler 2011; Lovett, Marshall & Carr 2006; Rutten et al. 2015), this subject remains little studied in the Cameroon Mountains.
The study of elevational gradients in different mountain systems in the tropics began as far back as 1800, with the work of Alexander von Humboldt and Charles Darwin. This early work led many scientists to centre research on these questions (Fischer et al. 2011), aiming to understand the distributional changes of species along different mountain gradients (Hemp 2002; Lovett et al. 2006; Rutten et al. 2015). In East Africa, the work of Lovett et al. (2006), Rutten et al. (2015) and Desalegn and Beierkuhnlein (2003) classified and delimited montane forest as starting at elevations from 700 to 1000 m above sea level (MASL).
The Cameroon Mountains have been termed by different authors as the Mountains of the Cameroons (Durrell 1954), Biafran Forests and Highlands (Burgess et al. 2007; Cronin et al. 2014), Cameroon Line (Nono et al. 2004) or Cameroon Volcanic Line (Ayonghe et al., 1999; Marzoli et al., 2000). This chain of volcanic and plutonic isolated mountains covers 40 877 km2 (Sainge 2016) and is isolated by ~2000 km from the Albertine Rift and by 1500 km-2000 km from the highlands of Liberia and Guinea (Alweny, Nsengiyumva & Gatarabirwa 2014; Burgess et al. 2007). This region ranks high among the biodiversity hotspots in the upper and lower Guinea forest and is thus considered to be of global conservation importance (Barthlott et al. 2005; Marchese 2015; Myers et al. 2000; Onana 2013; White 1983).
The Rumpi Hills Forest Reserve (RHFR) covers much of an important patch of wet montane forest in the continental part of the Cameroon Mountains that is considered a critical site for biodiversity conservation (Birdlife International 2016; Oates, Bergl & Linder 2004). Despite its high biodiversity potential, RHFR has received little attention in terms of detailed botanical and ecological surveys, when compared to surrounding mountain masses, such as Mt Nta Ali (Achoundong 1995; Thomas 1996), Mt Kupe (Cheek et al. 2004; Tchiengué 2004), the Bakossi National Park and Mt Mwanenguba (Cheek et al. 2004; Tchouto & Ebwekoh 1999), Mt Nlonako (Kenfack 2001), Mt Etinde (Tchouto 1995; Thomas & Cheek 1992), the Lebialem Highlands (Harvey, Tchiengué & Cheek 2010), Mt Oku (e.g. Cheek et al. 2000; Maisels & Forboseh 1997), Mt Cameroon (Cable & Cheek 1998; Tchouto et al. 1999) and Tchabal Mbabo (Chapman et al. 2004; Thomas & Thomas 1996). Understanding the Rumpi Hills ecosystem will thus allow future detailed comparisons across the region, as well as identification of the crucial conservation elements represented.
A few brief botanical collections were made in the Rumpi Hills area between 1976 and 1984, with a total of 57 botanical specimens collected by D. Dang, R. Letouzey, S. Polhill, B. Satabie and D. Thomas (National Herbarium of Cameroon database). It was not until 1996 that Thomas (1996) did a more intensive, rapid botanical survey of the area. Later, during 2000-2004, brief reconnaissance trips were made by D. Thomas, D. Kenfack and M. Sainge, during which 68 specimens were collected and deposited at the Missouri Botanical Garden Herbarium and the National Herbarium of Cameroon. The RHFR and surrounding areas are threatened by encroachment from both small farm estates and large-scale agro-industrial companies (Kupsch, Bobo & Waltert 2014).
In this study, we aimed to characterise vegetation patterns across the RHFR and to present a first detailed assessment of its vegetation structure and composition. This study is one element in a longer-term effort to assemble an understanding of the flora of the region. The specific objectives of this study were thus to understand the composition, structure and patterns in the RHFR along an elevational gradient.
Methods
Study area
The Rumpi Hills Forest Reserve lies near the south-western extreme of the Cameroon Mountain range, in Ndian Division, South-West Region, Cameroon. It stretches across latitudes 4.6°N - 5.0°N and longitudes 8.8°E - 9.4°E, with an elevational range of 50 m - 1778 m. It covers an area of 458 km2 (Forestry Ordinance 51 1941). Data were collected in clusters of 1-ha sampling plots: southern plots (numbers 1-8) were located on level terrain at elevations of 50 m - 200 m; northern plots (numbers 18-25) on fairly level terrain at 400 m - 600 m; four plots (numbers 14-17) on basaltic rock at 250 m - 300 m; and eastern plots (numbers 9-13) on undulating terrain at 1200 m - 1778 m (Figure 1).
The climate of the RHFR is typical of equatorial Cameroon, being hot and humid, with two distinct seasons: dry (December to March) and wet (April to November). An annual total rainfall of 5000 mm has been reported for the reserve (Nembot & Tchanou 1998). Temperature fluctuates with elevation, with the coldest temperatures at the top of Mount Rata; although no climate station is located in this area, Nembot and Tchanou (1998) reported a mean temperature of 22°C for the reserve. This reserve forms a topographic platform for different river sources that supply the Chad, Benue, Sanaga, Congo and Manyu rivers (Ngwa 1978). Rivers originating in this reserve flow in five directions: north into Lake Chad via the Logone River; northwest via the Benue River, the Kimbi River and the Katsina Ala River; southwest into the Gulf of Guinea via the Ndian (Moriba), Moko, Meme, Mungo and Wouri rivers; southeast via the Kadei River, a tributary of the Congo River, and west into Nigeria via the Munaya and Mbo rivers.
The reserve per se is free from human settlements, as no villages are located within its core area. Twelve villages are within 1 km - 3 km of the reserve margins: Matamani in the northwest; Mata in the north; Madie and Dikome Balue in the east; Munyange and Nalende in the south; Mbange, Bossunga, Motindi and Lipenja Mukete in the west; and Meka and Besingi in the northwest.
Field sampling
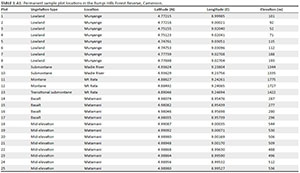
A reconnaissance survey based on topographic and vegetation maps of the reserve (Letouzey 1985) was carried out to identify homogeneous areas of putatively different vegetation types (Sainge & Cooper 2014). Data collection was done from February to June 2015, using 25 1-ha plots, which were placed based on accessibility to sample different vegetation types and elevations (Figure 1). Each plot measured 500 m long × 20 m wide and was subdivided into 25 quadrats of 20 m × 20 m. For each plot, global positioning system (GPS) coordinates were recorded for the four corners, including start and end points (Appendix 1), via careful, repeated measures to assure accuracy (coordinates of these permanent plots are available at http://hdl.handle.net/1808/25180). In each plot, all trees and lianas with diameter at breast height (dbh, 1.3 m above ground) of ≥ 10 cm were identified, measured with a diameter tape, tagged, recorded and mapped using their GPS coordinates. Smaller trees, shrubs and lianas (dbh < 10 cm) were sampled and measured with calipers in 10 m × 10 m quadrats located in every fifth 20 m × 20 m quadrat in each 1-ha plot. The forest was divided into four vertical strata: trees < 10 cm dbh as understory, 10 cm - 30 cm dbh as mid-canopy, 30 cm - 60 cm dbh as canopy and ≥ 60 cm dbh as emergent species. Finally, we recorded non-plot-based observational data (i.e. general plant collections) to detect and include species not present on the standardised plots.
Taxonomy and plant identification
In the field, plant identification was done using five-letter codes, including the first three letters of the genus and the first two letters of the species. In cases where the genus and species were not known or only the genus or family was known, arbitrary codes were generated to represent morphospecies. For unknown species (those that could not be identified and those partly identified or with doubtful identification), herbarium specimens were collected, labelled, pressed and dried for proper identification at the National Herbarium of Cameroon, in Yaoundé. Flowers and fruits were collected when the species was possibly new to science, endangered or endemic to the area. Identification in the herbarium was accomplished by comparing and matching specimens with existing collections and available floras and monographs. Plant classification followed species lists in the Angiosperm Phylogeny Group (APG III, 2009), with the Papilionaceae, Caesalpiniaceae and Mimosaceae merged into Fabaceae, and Sterculiaceae, Tiliaceae and Malvaceae merged into Malvaceae (APG III 2009; Judd et al. 1999).
Data analysis
Correlation analysis was used to assess the relationships between numbers of species and elevation, in PAST, version 2.17 (Hammer, Harper & Ryan 2001). Individuals not identified to the species level (1271 individuals with dbh ≥ 10 cm, 10.6%) and singletons (57 individuals, 0.5%) were excluded from inventory completeness calculations. Thus, 10 709 fully identified individual trees (dbh ≥ 10 cm, 88.9%) of 311 species were used in the analysis. Classification of the vegetation was achieved using two-way indicator species analysis (TWINSPAN; Hill 1979). Detrended correspondence analysis (DCA; Hill & Gauch 1980) was used to examine relationships between vegetation types and elevation, via PC-ORD for Windows, version 5.10 (McCune & Mefford 2006). Forest structure and composition were described using basal area, relative density, relative basal area, relative frequency and importance value index (Dallmeier 1992). Inventory completeness was assessed using EstimateS version 9.1.0 (Colwell 2013: http://purl.oclc.org/estimates), via the Chao2 estimator of expected species richness (Sexp), which is calculated from the number of species actually known from the site (Sobs) and frequency of detection of rare species; completeness was calculated as Sobs/Sexp, where Sexp = Sobs + a2/2b, a is the number of species detected only once and b is the number of species detected exactly twice. Completeness is then calculated as Sobs/Sexp.
Results
Species accumulation and inventory completeness
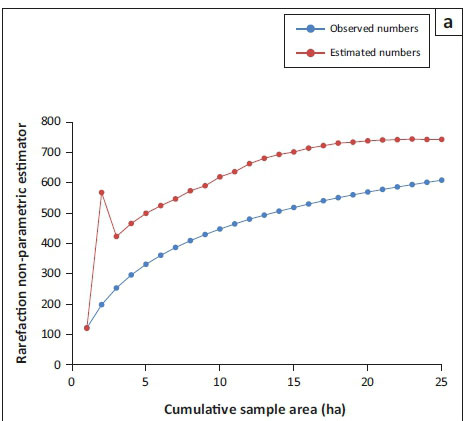
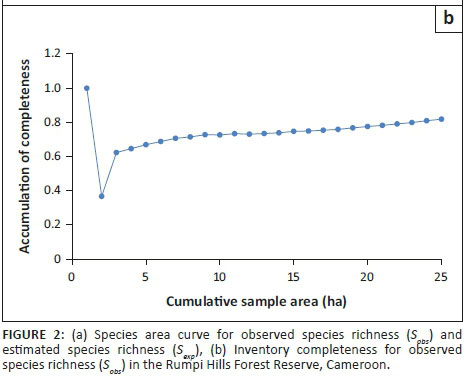
Inventory completeness varied considerably among plots, from a low of 0.36 in lowland plot 2 to 1.0 in lowland plot 1, with an overall mean of 0.73 (Figure 2a and b).
Composition and floristic structure
We recorded 4086 individual trees in lowland evergreen rainforest, 3600 in mid-elevation evergreen forest, 1831 in lowland evergreen rainforest on basalt rocks, 1191 in submontane forest, 1066 in montane cloud forest and 263 in transitional forest. For all vascular plants ≥ 10 cm, the highest mean number of trees per hectare (596) was recorded in the submontane forest (range 542-649), whereas the lowest mean tree number (263) was obtained in the transitional submontane forest (Table 1). The mean number of shrubs per 0.05 ha varied as follows: 180 shrubs/0.05 ha in lowland evergreen rainforest (range 140-212) and 71/0.05 ha in montane cloud forest (59-83). The mean number of lianas ranged from no lianas in montane or transitional forest at dbh ≥ 10 cm to 6 lianas per hectare (range 0-12) in lowland evergreen rainforest (Table 2).
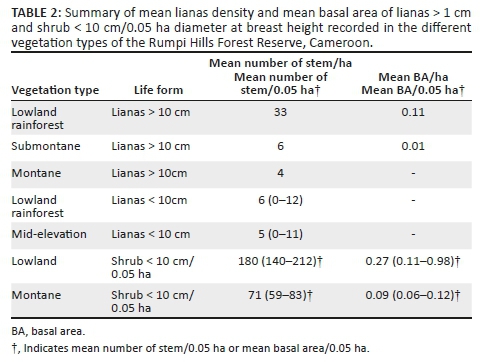
Mean basal area ranged from 37.5 m2 ha-1 in lowland evergreen rainforest (range 28.4 m2 ha-1 - 44.2 m2 ha-1) to 34.4 m2 ha-1 in montane cloud forest (34.3 m2 ha-1 - 34.5 m2 ha-1) (Table 1), for trees ≥ 10 cm dbh. For shrubs < 10 cm dbh, mean basal areas were low, ranging from 0.27 m2 ha-1 in lowland evergreen rainforest (range 0.11 m2 ha-1 - 0.98 m2 ha-1) to 0.09 m2 ha-1 in montane cloud forest (0.06 m2 ha-1 - 0.12 m2 ha-1). Lianas ≥ 10 cm dbh ranged from basal areas of 0.11 m2 ha-1 in lowland evergreen rainforest to 0.01 m2 ha-1 in submontane forest (Table 2).
Species composition by family for trees ≥ 10 cm dbh was as follows: Fabaceae, 954 individuals of 58 species; Rubiaceae, 310 individuals of 35 species; Annonaceae, 422 individuals of 26 species; and Sapotaceae (385 individuals) and Malvaceae (381 individuals), with 19 species each. Importance in terms of family (importance value index) for the 15 most common families was highest in Lecythidaceae (39.8), Fabaceae (20.9), Phyllanthaceae (19.9) and Malvaceae (13.7) and lowest in Sapotaceae (6.3), Ebenaceae (5.3), Sapindaceae (4.7) and Melastomataceae (1.8). The most broadly distributed family was Fabaceae, occurring in 390 of 625 quadrats sampled, whereas the rarest families in terms of number of species and abundance were Dilleniaceae, Malpighiaceae, Erythroxylaceae and Lepidobotryaceae, each represented by single individuals.
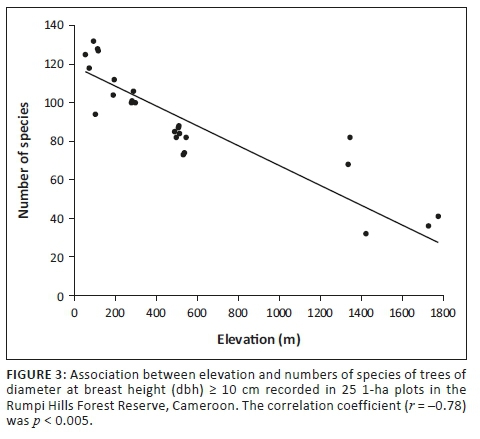
Lower-elevation vegetation types held many more species than high-elevation vegetation types. The linear multivariate model revealed a strong significant negative relationship between species richness and elevation (r = -0.783, p < 0.05; Figure 3), with numbers declining from 117.5 species (94-132 species) in lowland rainforest to 38.5 species (36-41 species) at high-elevation sites (Table 1). In all, 16 761 individuals of trees, shrubs and woody lianas were recorded in 25 1-ha plots across the RHFR.
Casual observational data provided records of an additional 254 individual plants in 62 families, 129 genera and 210 species. Indeed, 132 species were recorded only outside of the standardised sampling plots and quadrats. A total of 109 individuals in 21 morphospecies (97 trees and shrubs and 12 lianas) could not be identified to species level and 21 individuals could not be identified even to family. The most common 15 families accounted for 62% of total individual abundance across all plots.
Species composition changed drastically between vegetation types, as only a few species occurred in all vegetation types: Bridelia grandis (Phyllanthaceae), Cola verticillata (Malvaceae), Sapium ellipticum (Euphorbiaceae) and Symphonia globulifera (Clusiaceae). At the other end of the spectrum, many species were recorded only in single vegetation types, such as Afrostyrax kamerunensis, Afzelia bipindensis, A. pachyloba, Alexis cf. cauliflora, Allanblackia gabonensis and Allophylus megaphyllus in lowland forest; Beilschmiedia gabonensis, Leptonychia lasiogyne, Maesa kamerunensis, M. lanceolata, Syzygium staudtii and Trichoscypha amplexicaulis in submontane forest; Alangium chinense, Alchornea floribunda and Elaeophorbia drupifera in transitional submontane forest and Acridocarpus macrocalyx and Allophylus grandifolius in mid-elevation evergreen forest.
Multivariate analysis
The 25 1-ha plots were classified in the TWINSPAN analysis into six groups at 50% similarity (Figure 4). Plots 1-8 corresponded to lowland evergreen forest (sensu Letouzey 1968, 1985), characterised by an abundance of Oubanguia alata (556 individual trees). Plots 14-17, grouped together, corresponded to lowland evergreen forest on basalt rocks that had abundant Crateranthus talbotii (103 individual trees). Plots 18-25 clustered together and can be termed mid-elevation forest, abundant in Strombosia grandifolia (324 individual trees) and Leonadoxa africana (192 individual trees). Plots 9-10 were in submontane forest abundant in Tabernaemontana ventricosa (102 individual trees), Cola verticilata (75 individual trees) and Dasylepis thomasii (68 individual trees). Plots 11-12 were in montane cloud forest abundant in Strombosia sp. (146 individual trees), Carapa oreophila (125 individuals) and Xylopia africana (121 individual trees). Lastly, Plot 13 was in transitional submontane forest rich in Macaranga sp. (56 individual trees), Trema orientalis (46 individual trees), Bridelia grandis (33 individual trees) and Pauridiantha viridiflora (31 individual trees).
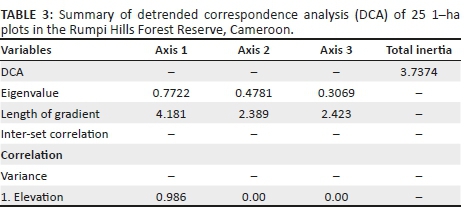
Our floristic dataset of 25 plots was also subjected to DCA analysis and plotted along Axes 1 and 2. Variation was expressed along Axis 1, with an eigenvalue of 0.772 and a gradient length of 4.183, which reflects high variation among vegetation types and species composition. Vegetation types 4, 5 and 6 (submontane, montane and transitional forest, respectively) separated toward the positive side of DCA Axis 1, whereas vegetation types 1, 2 and 3 (lowland, basalt and mid-elevation, respectively) separated toward the negative end (Figure 5). DCA Axis 2 showed a weaker eigenvalue of 0.478, with a gradient length of 2.389 (Table 3). Figure 5 shows patterns suggesting that vegetation types 1-3 are more closely related than vegetation types 4-6.
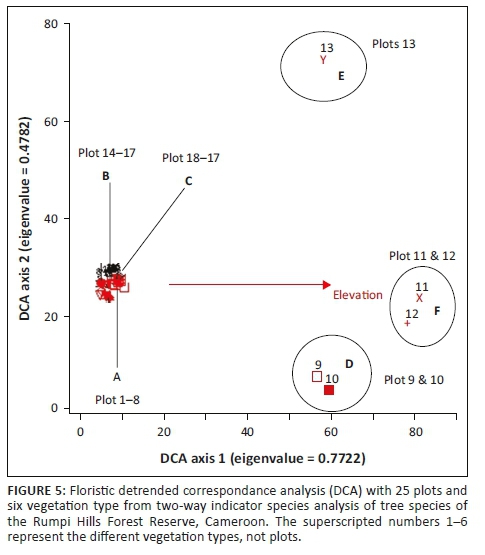
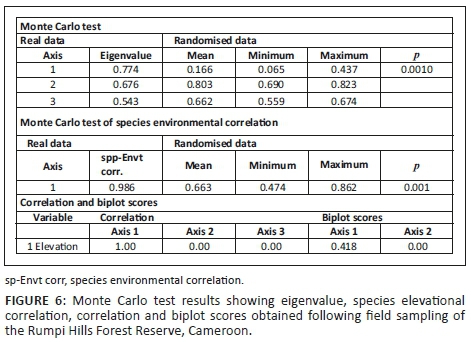
A high species-environment correlation for Axis 1 indicates a strong association between vegetation type and elevation, which can be verified from the biplot record (Figure 6). A Monte Carlo permutation test (998 runs) with an eigenvalue of Axis 1 and significant at p < 0.001 confirmed the strong relationship between species composition and elevation (Figure 6).
Vegetation patterns
Lowland evergreen rainforest abundant in Oubanguia alata Baker f.
This vegetation type occurs along the southern edge of RHFR, mainly at elevations below 250 m. The vegetation is intact, evergreen and continuous from the ground layer of herbaceous plants in dense undergrowth to emergent tree species. The canopy is more or less continuous, with only a few emergents. In all, 4086 trees were recorded, belonging to 51 families, 174 genera and 291 species. Seven species were not fully identified and 2 individuals were not identified even to family. Shrubs with dbh < 10 cm totalled 1065 individual trees in 44 families, 126 genera and 208 species. Only one individual of this group of plants was not identified to genus or species. Lianas of < 10 cm dbh included 7 individuals in 3 species, 2 genera and 2 families.
Dominant tree families in lowland evergreen rainforest were Fabaceae (61 species), Annonaceae (26 species) and Euphorbiaceae, Malvaceae and Phyllanthaceae, with 19 species each. Frequent genera included Cola (14 species), Diospyros (13 species) and Drypetes and Trichoscypha (10 species each). TWINSPAN analysis presented Oubanguia alata (556 individuals, or 13.6% of total individuals in this vegetation type), Protomegabaria stapfiana (263 individuals, 6.4%) and Korupodendron songweanum (164 individuals, 4%) as dominant species in this vegetation type.
Some emergent and upper canopy species were 30 m - 55 m tall, with huge buttresses that spanned 3 m - 10 m on the forest floor and trunk diameters of up to 2.5 m. Such tree species included Microberlinia bisulcata, Irvingia gabonensis, Desbordesia glaucescens, Pycnanthus angolensis, Saccoglottis gabonensis, Omphalocarpum elatum and Engomegoma gordonii. The canopy, 20 m - 35 m tall, was composed of tree species such as Tetraberlinia bifoliolata, Korupodendron songweanum, Santiria balsamifera, Eriocoelum macrocarpum and Pellegriniodendron diphyllum.
The mid-story was made up of small trees, mostly immature emergent and canopy trees, of ~10 m tall, composed of Uvariopsis bakeriana, Craterispermum aristatum, Napoleonaea talbotii, Deinbollia angustifolia, Phyllobotryon spathulathum and Gaertnera bieleri. The ground layer, composed of herbaceous species, seedlings of the aforementioned layers, lianas and creepers, was dominated by Deinbollia angustifolia, Impatiens niamniamensis, Costus afer, Anubias barteri, Begonia quadrialata and Palisota hirsuta. Seven large woody liana species were recorded in this forest type, of which Connarus staudtii, Dichapetalum affine, Rhaphiostylis beninensis, Strychnos tricalysioides and Santalinoides afzelii were the most common.
Lowland evergreen rainforest on basalt rocks abundant in Crateranthus talbotii Baker f.
This vegetation type occurs along the western edge of the RHFR, at elevations of 250 m - 300 m and dominated by Crateranthus talbotii (103 individuals, 5.6%), Trichilia prieureana (65 individuals, 3.5%) and Anthonotha macrophylla (64 individuals, 3.4%). This vegetation type is characterised by trees, shrubs and lianas with dbh < 22 cm and is made up of patches of huge basalt rocks. In all, 1831 trees with dbh ≥ 10 cm were recorded in this vegetation type, in 39 families, 122 genera and 175 species. Two tree species could not be identified to genus. Shrubs with dbh < 10 cm amounted to 40 families, 85 genera and 119 species. This forest type was characterised by a rich diversity of Fabaceae (234 individuals of 21 species), Rubiaceae (29 individuals of 11 species), Phyllanthaceae (98 individuals of 10 species) and Anacardiaceae (72 individuals of 10 species).
Mid-elevation evergreen forest abundant in Strombosia grandifolia Hook f.
This vegetation type occurs in the northern parts of the RHFR, at elevations of 300 m - 800 m, and is dominated by Strombosia grandifolia (324 individuals) and Leonadoxa africana (192 individuals). We sampled 3600 trees in 42 families, 119 genera and 176 species and 1153 shrubs in 41 families, 98 genera and 150 species. The most frequent shrub genera were Strombosia, Leonodoxa, Trichilia and Tabernaemontana; the most common species were Strombosia grandifolia, Oubanguia alata, Trichilia prieureana, Tabernaemontana brachyantha and Mammea africana.
Submontane forest abundant in Tabernaemontana ventricosa Hochst. ex A.DC.
This forest type was found along the eastern edge of the RHFR, at 800 m - 1600 m elevation. It occurs close to the villages of Dikome Balue, Madie and Iyombo and along the western edge of the reserve around Mbange, Bossunga, Boa Yenge and Motindi villages. The Rumpi Hills Forest Reserve submontane forest occurs on steep cliffs and inaccessible slopes. We sampled 1191 trees of 38 families, 75 genera and 98 species; shrubs totalled 240 individuals in 32 families, 49 genera and 60 species. This forest type was rich in Rubiaceae (9 species), Euphorbiaceae (7 species) and Meliaceae (6 species) for trees; common species included Tabernaemontana ventricosa, Dasylepis thomasii, Cola verticillata, Diospyros cinnabarina and Garcinia conrauana. The submontane forest forms part of a mosaic of forest and grassland savanna above 1300 m - 1600 m.
The canopy here is relatively short (20 m - 25 m tall), with only a few tall trees, such as Cylicomorpha solmsii, Eriocoelum macrocarpum, Guarea cedrata and Sapium ellipticum, reaching 30 m - 35 m tall. Three species of large woody lianas were recorded: Dichapetalum heudelotii, Salacia pyriformis and an unidentified species.
Transitional submontane forest abundant in Trema orientalis (L.) Blume
This vegetation type is a mosaic of forest and grassland occurring along the eastern edge of the RHFR at 1300 m - 1600 m, close to Dikome Balue, and is characterised by low diversity. We sampled 263 individual trees in 20 families, 31 genera and 32 species with dbh ≥ 10 cm and 61 individual trees in 16 families, 22 genera and 24 species with dbh < 10 cm. Dominant tree families with dbh ≥ 10 cm were Euphorbiaceae and Rubiaceae (4 species each); all other families were represented by only 1-2 tree species. Dominant genera were Macaranga, Trema, Pauridiantha, Bridelia and Margaritaria; most common species included Macaranga sp., Trema orientalis and Pauridiantha viridiflora. Rare species recorded here included Thunbergia affinis, Alangium chinense, Trichoscypha preussii, Monodora myristica and Uvariastrum pynaertii.
Montane cloud forest abundant in Carapa oreophila Kenfack
Montane forest is found on the eastern edge of the RHFR, at elevations > 1600 m, up to the summit of Mount Rata (~1778 m). We sampled 1066 individual trees with dbh ≥ 10 cm, in 25 families, 38 genera and 48 species, and 146 individual trees with dbh < 10 cm, in 19 families, 24 genera and 31 species. This forest was characterised by species of Rubiaceae (6 species), Clusiaceae (5 species) and Salicaceae (4 species). Most abundant species included Strombosia sp. (146 individuals, 13.7%), Carapa oreophila (125 individuals, 11.7%), Xylopia africana (121 individuals, 11.4%) and Dasylepis thomasii (91 individuals, 8.5%). The upper canopy layer was 20 m - 25 m tall, with most species covered with numerous species of epiphytes (orchids, mosses, lichens, liverworts, bryophytes and Piperaceae). Understory vegetation was sparse. Woody lianas were scarce, with only two species recorded (Dichapetalum rudatisii and an unknown species).
Discussion
The diverse and heterogeneous vegetation structure and composition in RHFR are likely related to the complex physical features, elevational differences (from 50 m to the top of Mount Rata) and climatic factors, such as the south-western monsoon (warm wet) winds that are weak in the dry season and strong in the wet season (Neba 1999; Ngwa 1978). Rainfall is highest in the south-western corner of the reserve; however, to date, no weather station has been installed to provide detailed climatic data for the reserve. We assume that, given its proximity to Korup National Park, with an average yearly rainfall of ~5 m (Chuyong, Newbery & Songwe 2000, 2004; Newbery & Gartlan 1996; Thomas et al. 2003), RHFR also has high rainfall; temperature within the reserve is variable, with temperatures lowest at the top of Mount Rata.
The full set of characteristics of RHFR (i.e. its enclaved nature, importance as a watershed and lack of human settlements) are indicators of conservation importance. This importance is emphasised by our findings of high tree, liana and shrub diversity (Table 1) and the large number of species of high conservation priority (Online Appendix 1), as well as the fact that it occurs in a recognised biodiversity hotspot (Barthlott et al. 2005; Marchese 2015; Myers et al. 2000; Onana 2013; White 1983).
Vegetation patterns and floristic composition
Multivariate analyses (TWINSPAN and DCA) showed strong influences of elevation on forest types. TWINSPAN analyses classified RHFR vegetation into six types. Letouzey (1985) recognised seven vegetation types in RHFR: the Atlantic Biafran forest; Atlantic littoral forest (which in the current study is classified as lowland forest); piedmont forest; degraded submontane forest; submontane forest; highly degraded evergreen forest (which was not sampled in the present study); and submontane grassland (also not sampled), as well as various combinations of fallow, grazed and human-inhabited areas (Figure 1).
The RHFR is part of the chain of mountains of Cameroon and Nigeria that includes the Cameroon Mountains and associated highland biomes (Burgess et al. 2007; Cronin et al. 2014). It forms part of the Lower Guinea Forest, with high levels of species richness and endemism (Barthlott et al. 2005; Burgess et al. 2007; Plumptre et al. 2007). The occurrence of a mosaic of forest and grassland on the upper slopes of Mount Rata, at elevations of 1300 m - 1600 m, is not surprising, as grassland savanna begins at 1500 m in the Takamanda Forest Reserve, in the South-West Region of Cameroon (Sunderland et al. 2003), and above 2000 m on Mount Cameroon (Richards 1963). Administratively, Mount Rata falls outside of the RHFR and yet represents the highest peak in the Rumpi Hills range.
Our results differ from patterns in lowland forest at other sites, like the Takamanda Forest Reserve (South-West Region, Cameroon), where lowland forest is dominated by Huaceae (Afrostyrax kamerunensis) and Irvingiaceae (Irvingia, Klainedoxa, Desbordosea) (Sunderland et al. 2003). However, the RHFR lowland forest showed the same dominance trends as the lowland forest in nearby Korup National Park (Thomas et al. 2003).
Forest structure changed from lowland evergreen forest (50 m - 200 m), with some trees 35 m - 55 m tall, to montane cloud forest (1778 m), with a lower and more even canopy 20 m - 25 m tall, comprising trees with branches covered by Piperaceae, Orchidaceae, ferns, liverworts, lichens and so on. Our results agree with Letouzey (1985) that RHFR is composed of different vegetation types and show that these vegetation types demonstrate impressive variation in structure, species composition and distribution. Furthermore, RHFR contains a distinct montane vegetation type, as detected and defined by the TWINSPAN analysis, at elevations above 1600 m. This result concurs with Vallèrié (1971) and Thomas (1984), who both classified upper montane forest as starting from 1600 m in the Cameroon Mountains region.
Elevation
The effect of elevation on the vegetation of the RHFR was pronounced, as it influences vegetation pattern, vegetation structure, species diversity and species composition of the area (Figure 4) across an elevational range of 50 m - 1778 m. We documented marked changes in species composition with elevation: lowland evergreen rainforest on basalt and lowland evergreen rainforest rich in O. alata were relatively richer in species than the other vegetation types. The inverse relationship between species richness and elevation recorded in RHFR was consistent with results obtained in many studies (Chuyong et al. 2011; Hamilton 1975; Henrik et al. 2006). Dauby et al. (2013) investigated tree diversity patterns in communities of evergreen forest trees in five landscapes of western Gabon and concluded that mean alpha and gamma diversities were much higher in the hilly region, with differences in elevation explaining a significant part of species turnover. Decreased alpha diversity with elevation within the hilly region could be associated with mass effects, which are expected to enrich valleys and slopes (Dauby et al. 2013). Overall, in RHFR lowland forest is characterised by large trees with huge buttresses and lianas; at high elevations, shrubs and tree branches are covered with bryophytes and vascular epiphytes and tree boles and leaves are covered by moss and liverworts, with a minimal liana population.
Our detailed sampling across vegetation types and elevations within and near the RHFR makes our data useful both for ecological understanding and for guiding management decisions. Given that our plots are permanent, with GPS-based outlines of each sampling plot (http://hdl.handle.net/1808/25180), the opportunity arises to repeat these censuses in the future (e.g. every 5-10 years) to understand the dynamics of the forest (Condit 1998). Such detailed monitoring would allow a far more nuanced understanding of the status and condition of these forests, as well as of the effects of global change on their composition and structure.
Conservation implications
Conservation prioritisations usually involve a number of factors for designing conservation areas, incorporating aspects of species composition, structure, vegetation patterns and socio-economic and cultural importance of the sites. Our study confirmed that RHFR is a rich site in terms of vegetation; analysis of species composition segregated the community into six vegetation types, some of which are found only outside of the administrative boundaries of the reserve (e.g. montane cloud forest). Our records of rare species, such as Deinbollia angustifolia, Korupodendron songweanum, Gambeya korupensis and Oubanguia alata in lowland forest; Crateranthus talbotii in lowland basalt forest; Rhaptopetalum geophylax and Cylicomorphia solmsii in submontane forest; and Carapa oreophila, Oncoba lophocarpa and Xylopia africana in montane forest, may upgrade the conservation interest in the reserve. Particular species occurred only in specific vegetation types or in a few adjacent types. Hence, protection of each of the different vegetation types, including the unique vegetation types at higher elevation on Mount Rata, is paramount for conservation.
Acknowledgements
Over the years, we have received generous support from the Rufford Small Grant Foundation (RSG); this work was supported by the first booster grant of RSG 16712-B. A complementary grant was also received from the Tropical Plant Exploration Group (TroPEG) Cameroon, and some support was provided by a grant to A.T.P. from the JRS Biodiversity Foundation. We are thankful to the Government of Cameroon for research permits. The first author expresses his gratitude to colleagues at TroPEG Cameroon. The chiefs and residents of Matamani, Munyange and Dikome Balue villages are thanked for their hospitality and support. The field assistants who toiled in the forest during data collection are highly appreciated, especially Mambo Peter Ekole, Motia Innocent, Motto Moses, Joseph Mulango, Hans Notto and Okere Frederick.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
M.N.S. was the project leader, responsible for project design and carried out all data collection. N.M.L. was part of the field survey team and made conceptual contributions. G.P.T.M and D.K. made conceptual contributions. F.N. and A.T.P. made conceptual contributions and edited and reviewed the manuscript.
References
Achoundong, G., 1995, 'Les formations submontagnardes du Nta-Ali au Cameroon', Bois et Forêts des Tropiques 243, 51-63. [ Links ]
Alweny, S., Nsengiyumva, P. & Gatarabirwa, W., 2014, Africa sustainable mountain development, Technical Report No.1, Albertine Rift Conservation Society (ARCOS), October 2014, Kampala, Uganda, p. 36. [ Links ]
Angiosperm Phylogeny Group III, 2009, 'An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III', Botanical Journal of the Linnean Society 161, 105-121. https://doi.org/10.1111/j.1095-8339.2009.00996.x [ Links ]
Ayonghe, S.N., Mafany, G.T., Ntasin, E. & Samalang, P., 1999, 'Seismically activated swarm of landslides, tension cracks and a rock fall after heavy rainfall in Bafaka, Cameroon', Natural Hazards 9, 13-27. https://doi.org/10.1023/A:1008041205256 [ Links ]
Barthlott, W., Mutke, J., Rafiqpoor, D., Kier, G. & Kreft, H., 2005, 'Global centers of vascular plant diversity', Nova Acta Leopoldina 92, 61-83. [ Links ]
BirdLife International, 2016, 'CM024: Mount Rata and Rumpi Hills Forest Reserve, viewed 01 May 2016, from http://www.birdlife.org [ Links ]
Burgess, N.D., Balmford, A., Cordeiro, N.J., Fjelda, J., Küper, W., Rahhek, C. et al., 2007, 'Correlations among species distributions, human density and human infrastructure across the high biodiversity tropical mountains of Africa', Biological Conservation 134, 164-177. https://doi.org/10.1016/j.biocon.2006.08.024 [ Links ]
Cable, S. & Cheek, M., 1998, The plants of Mount Cameroon. A conservation checklist, Royal Botanic Gardens, Kew, London, UK, p. 198. [ Links ]
Campbell, P., Rivera, P., Thomas, D., Bourobou-Bourobou, H., Nzabi, T., Alonso, A. et al., 2006, 'Floristic structure, composition and diversity in an equatorial forest in Gabon', Bulletin of the Biological Society of Washington 12, 253-273. [ Links ]
Chapman, H.M., Bekker, R., Barnard, J. & Shi, G., 2004, The botany of Tchabal Mbabo, Cameroon. A contribution towards the Nigeria and Cameroon Transboundary initiative, University of Canterbury, Christchurch, New Zealand, p. 1. [ Links ]
Cheek, M., Onana, J.M. & Pollard, B.J., 2000, The Plants of Mount Oku and the Ijim Ridge, Cameroon, Royal Botanic Garden, Kew, Richmond, p. 211. [ Links ]
Cheek, M., Pollard, B.J., Darbyshire, L., Onana, J.M. & Wild, C., 2004, The plants of Kupe, Mwanenguba and the Bakossi Mountains, Cameroon, Conservation checklist, Royal Botanic Garden, Kew, Richmond, p. 508. [ Links ]
Chuyong, G.B., Newbery, D.M. & Songwe, N.C., 2000, 'Litter nutrients and retranslocation in a Central African rainforest dominated by ectomycorrhizal trees', New Phytologist 148, 493-510. https://doi.org/10.1046/j.1469-8137.2000.00774.x [ Links ]
Chuyong, G.B., Newbery, D.M. & Songwe, N.C., 2004, 'Rainfall input, throughfall and stemflow of nutrients in a Central African rain forest dominated by ectomycorrhizal trees', Biogeochemistry 67, 73-91. https://doi.org/10.1023/B:BIOG.0000015316.90198.cf [ Links ]
Chuyong, G., Kenfack, D., Harms, K., Thomas, D., Condit, R. & Comita, L., 2011, 'Habitat specificity and diversity of tree species in an African wet tropical forest', Plant Ecology 212, 1363-1374. https://doi.org/10.1007/s11258-011-9912-4 [ Links ]
Colwell, R.K., 2013, Estimates: Statistical estimation of species richness and shared species from samples, Version 9.1.0, viewed 03 November 2016, from http://purl.oclc.org/estimates. [ Links ]
Condit, R., 1998, Tropical Forest Census Plots: Methods and results from Barro Colorado Island (BCI), Panama and a comparison with other plots, Springer-Verlag, Berlin, p. 217. [ Links ]
Cronin, D.T., Libalah, M.B., Bergl, R.A. & Hearn, G.W., 2014, 'Biodiversity and conservation of tropical montane ecosystems in the Gulf of Guinea, West Africa', Arctic, Antarctic and Alpine Research 46, 891-904. https://doi.org/10.1657/1938-4246-46.4.891 [ Links ]
Dallmeier, F., 1992, Long-term monitoring of biological diversity of tropical forest areas: Methods for the establishment and inventory of permanent plots, United Nation Educational Scientific and Cultural Organization (UNESCO), Paris, p. 72. [ Links ]
Desalegn, W. & Beierkuhnlein, C., 2010, 'Plant species and growth forms richness along altitudinal gradients in the southwest Ethiopian highlands', Journal of Vegetation Science 21, 617-626. https://doi.org/10.1111/j.1654-1103.2010.01177.x [ Links ]
Durrell, G., 1954, The Bafut Beagles, Penguin Books, London, p. 125. [ Links ]
Dauby, G., Hardy, O.J., Leal, M., Breteler, F. & Stévart, T., 2013, 'Drivers of tree diversity in tropical rain forests: New insights from a comparison between littoral and hilly landscapes of Central Africa', Journal of Biogeography 41, 574-586. https://doi.org/10.1111/jbi.12233 [ Links ]
Fischer, A., Blaschke, M. & Bassler, C., 2011, 'Altitudinal gradients in biodiversity research: The state of the art and future perspectives under climate change aspects', Waldokologie, Landschaftsforschung und Naturschutz 11, 35-47. [ Links ]
Forestry Ordinance, 51, 1941, Establishment of the Rumpi Hills Native Authority Forest Reserve, Forestry Archive Buea, Buea, Cameroon, p. 5. [ Links ]
Gentry, A.H., 1988, 'Changes in plant community diversity and floristic composition on environmental and geographical gradients', Annals of the Missouri Botanical Garden 75, 1-34. https://doi.org/10.2307/2399464 [ Links ]
Gonmadje, C.F., Doumenge, C., McKey, D. & Sonké, B., 2011, 'Tree diversity and conservation value of Ngovayang's lowland forest, Cameroon', Biodiversity and Conservation 20, 2627-2648. https://doi.org/10.1007/s10531-011-0095-z [ Links ]
Hamilton, A.C., 1975, 'A quantitative analysis of altitudinal zonation in Uganda forests', Vegetation 30, 99-106. https://doi.org/10.1007/BF02389611 [ Links ]
Hammer, Ø., Harper, D.A.T. & Ryan, P.D., 2001, 'PAST: Paleontological Statistics Software package for education and data analysis', Palaeontologia Electronica 4, 1-9. [ Links ]
Harvey, Y., Tchiengué, B. & Cheek, M., 2010, The plants of Lebialem Highlands, Cameroon: A conservation checklist, Royal Botanic Gardens, Kew, p. 170. [ Links ]
Hemp, A., 2002, 'Ecology of the pteridophytes on the southern slopes of Mt Kilimanjaro-1. Altitudinal distribution', Plant Ecology 159, 211-239. https://doi.org/10.1023/A:1015569125417 [ Links ]
Henrik, B.H., Moen, J., Virtanen, R., Grytnes, J.A., Oksanen, L. & Angerbjörn, A., 2006, 'Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities', Journal of Vegetation Science 17, 37-46. https://doi.org/10.1111/j.1654-1103.2006.tb02421.x [ Links ]
Hill, M.O., 1979, TWINSPAN-a Fortran program for arranging multivariate data in an ordered two-way table of classification of individuals and attributes, Cornell University, Ithaca, NY, p. 90. [ Links ]
Hill, M.O. & Gauch, H.G., 1980, 'Detrended correspondence analysis, an improved ordination technique', Vegetatio 42, 47-58. https://doi.org/10.1007/BF00048870 [ Links ]
Imani, G., Zapfack, L., Kalume, J., Riera, B., Cirimwami, L. & Boyemba, F., 2016, 'Woody vegetation groups and diversity along the altitudinal gradient in mountain forest: Case study of Kahuzi-Biega National Park and its surroundings, DRC', Journal of Biodiversity and Environmental Sciences 8, 134-150. [ Links ]
Judd, S.W., Campbell, S.C., Kellogg, A.E. & Stevens, F.P., 1999, Plant systematics: A phylogenetic approach, Sinauer Associates, Sunderland, MA, p. 464. [ Links ]
Kenfack, D., 2001, Preliminary botanical survey of Mt Nlonako, Makombe, Ebo and Lake Ossa, Report to the Cross-Sanaga - Bioko Coastal Forest Project, Worldwide Fund for Nature-Coastal Forest Program, Cameroon, p. 84. [ Links ]
Kupsch, D., Bobo, K.S. & Waltert, M., 2014, Biodiversity, carbon stock and market value assessment for the Sustainable Oil (SGSOC) project area, Southwest Region, Cameroon, Report submitted to World Wide Fund for Nature (WWF), Georg-August-Universität Göttingen, Göttingen, Germany, p. 41. Technical Report. [ Links ]
Letouzey, R., 1968, 'Etude phytogéographique du Cameroon. Encyclopedie Biologique', Paris, Lechevalier 49, 508. [ Links ]
Letouzey, R., 1985, 'Carte phytogéographique du Cameroun', Vol. 1-5, Institut de la Carte Internationale de la Végétation, Toulouse-France, p. 240. [ Links ]
Lovett, J.C., Marshall, A.R. & Carr, J., 2006, 'Changes in tropical forest vegetation along an altitudinal gradient in the Udzungwa Mountains National Park', African Journal of Ecology 44, 478-490. https://doi.org/10.1111/j.1365-2028.2006.00660.x [ Links ]
Lutz, J.A., Larson, A.J., Swanson, M.E. & Freund, J.A., 2012, 'Ecological Importance of large-diameter trees in a temperate mixed-conifer forest', PLoS One 7, 1-15. https://doi.org/10.1371/journal.pone.0036131 [ Links ]
Maisels, F.M. & Forboseh, P., 1997, Vegetation survey, Report to Kilum-Ijim Forest Project, Oku, Cameroon, p. 51. [ Links ]
Marchese, C., 2015, 'Biodiversity hotspots: A shortcut for a more complicated concept', Global Ecology and Conservation 3, 297-309. https://doi.org/10.1016/j.gecco.2014.12.008 [ Links ]
Marzoli, A., Piccirillo, E.M., Renne, P.R., Bellieni, G., Iacumin, M., Nyobe, J.B. & Tongwa, A.T., 2000, 'The Cameroon Volcanic Line revisited: Petrogenesis of continental basaltic magmas from lithospheric and asthenospheric mantle sources', Journal of Petrology 41, 87-109. https://doi.org/10.1093/petrology/41.1.87 [ Links ]
McCune, B. & Mefford, M.J., 2006, PC-ORD multivariate analysis of rcological data, Version 5.10, MjM Software Design, Gleneden Beach, OR, p. 28. [ Links ]
Myers, N., Mittermeier, R.A., Mittermeier, C.G., da Fonseca, G.A.B. & Kent, J., 2000, 'Biodiversity hotspots for conservation priorities', Nature 403, 853-858. https://doi.org/10.1038/35002501 [ Links ]
Neba, A., 1999, Modern geography of the Republic of Cameroon, 3rd edn., Neba Publishers, Bamenda, Cameroon, p. 269. [ Links ]
Neelo, J., Teketay, D., Kashe, K. & Masamba, W., 2015, 'Stand structure, diversity and regeneration status of woody species in open and enclosed dry woodland sites around Molapo farming area of the Okavango delta, northeastern Botswana', Open Journal of Forestry 5, 313-328. https://doi.org/10.4236/ojf.2015.54027 [ Links ]
Nembot, T.F. & Tchanou, Z., 1998, 'La Gestion des ecosystems forestriers du Cameroun a l'aube de l'an 2000', vol. 2, Monographies des Sites Critiques, International Union for Conservation of Nature (IUCN), Yaounde, Cameroun, p. 283. Technical Report. [ Links ]
Newbery, D.M. & Gartlan, J.S., 1996, 'A structural analysis of rain forest in Korup and Douala-Edea, Cameroon', Proceedings of the Royal Society of Edinburgh 104B, 177-224. [ Links ]
Ngwa, J.A., 1978, A new geography of Cameroon, Longman Group Limited, Essex, United Kingdom, p. 151. [ Links ]
Nono, A., Njonfang, E., Dongmo, A.K., Nkouathio, D.G. & Tchoua, F.M., 2004, 'Pyroclastic deposits of the Bambouto Volcano (Cameroon Line, Central Africa): Evidence of an initial strombolian phase', Journal of African Earth Sciences 39, 409-414. https://doi.org/10.1016/j.jafrearsci.2004.07.026 [ Links ]
Noumi, E., 2013, 'Floristic inventory of woody species in the Manengouba Mountains Forest Cameroon', Journal of Biology and Life Science 4, 282-309. https://doi.org/10.5296/jbls.v4i2.4014 [ Links ]
Oates, J.F., Bergl, R.A. & Linder, J.M., 2004, Africa's Gulf of Guinea forests: Biodiversity patterns and conservation priorities, Advances in Applied Conservation Biology (6), Conservation International, Washington, DC, p. 90. [ Links ]
Onana, J.M., 2013, 'Environnement biophysique', in J.M. Onana (ed.), Synopsis des espèces végétales, vasculaires endémiques et rares du Cameroun: Checkliste pour la conservation et la gestion durable de la biodiversité, Flore du Cameroun 40, pp. 6-23, Ministère de la Recherche Scientifique et de l'Innovation, Yaoundé [ Links ].
Plumptre, A.J., Davenport, T., Behangana, M., Kityo, R., Ndomba, E., Nkuutu, D. et al., 2007, 'The Biodiversity of the Albertine Rift', Biological Conservation 134, 178-194. https://doi.org/10.1016/j.biocon.2006.08.021 [ Links ]
Richards, P.W., 1963, 'Ecological notes on West African vegetation III. The upland forests of Cameroon Mountains', Journal of Ecology 51, 529-554. https://doi.org/10.2307/2257746 [ Links ]
Richter, M., 2008, 'Tropical mountain forests: Distribution and general features', Biodiversity and Ecology Series 2, 7-24. [ Links ]
Rutten, G., Ensslin, A., Hemp, A. & Fischer, M., 2015, 'Vertical and horizontal vegetation structure across natural and modified habitat types at Mount Kilimanjaro', PLoS One 10(9), e0138822. https://doi.org/10.1371/journal.pone.0138822 [ Links ]
Sainge, M.N., Onana, J.-M., Nchu, F., Kenfack, D. & Peterson, A.T., 2017, 'Botanical sampling gaps across the Cameroon Mountains', Biodiversity Informatics 12, 76-83. [ Links ]
Sainge, M.N., 2016, Patterns of distribution and endemism of plants in the Cameroon Mountains: A case study of protected areas in Cameroon, Rumpi Hills Forest Reserve (RHFR) and the Kimbi Fungom National Park (KFNP), Final report to Rufford Small Grant Foundation, Tropical Plant Exploration Group (TroPEG), Buea, Cameroon, p. 171 [ Links ]
Sainge, M.N. & Cooper, J.C., 2014, Reconnaissance survey of the biodiversity of the Cameroon Mountains, Cameroon Mountains Research Project. Tropical Plant Exploration Group (TroPEG), Buea, Mundemba, Cameroon, p. 55. [ Links ]
Sunderland, T.C.H., Comiskey, J.A., Besong, S., Mboh, H., Fonwebon, J. & Dione, M.A., 2003, 'Vegetation Assessment of Takamanda Forest Reserve, Cameroon', in J.A. Comiskey, T.C.H. Sunderland & J.L. Sunderland-Groves (eds.), Takamanda: The Biodiversity of an African rainforest, Smithsonian Institution/Man and the Biosphere #8, p. 192, Smithsonian Institution, Washington, DC. [ Links ]
Tchiengué, B., 2004, 'Etude Ecologique et Floristique de la vegetation d'un massif de la ligne du Cameroun: Le Mont Koupe', Thèse, Maitre és Sciences, Université de Yaounde 1, p. 235. [ Links ]
Tchouto, M.G.P., 1995, 'The vegetation of the proposed Etinde Rain Forest Reserve, Mount Cameroon and its conservation', MSc thesis, University of Edinburgh. [ Links ]
Tchouto, M.G.P., 2004, 'Plant diversity in a Central Africa rain forest: Implications for biodiversity conservation in Cameroon', PhD thesis, Department of Plant Sciences, Biosystematics Group, Wageningen University, The Netherlands, p. 208. [ Links ]
Tchouto, P. & Ebwekoh, M.O., 1999, A participatory Rapid Biodiversity Survey of the Muanemguba Mountain Forest, Final report to Centre for Environment and Rural Transformation (CERUT), Limbe, Cameroon, p. 54. [ Links ]
Tchouto, M.G.P., Edwards, I., Cheek, M., Ndam, N. & Acworth, J., 1999, 'Mount Cameroon cloud forest', in J. Timberlake & S. Kativu (eds.), African plants: Biodiversity, taxonomy and uses, Royal Botanic Gardens, Kew, London, UK. pp. 263-277. [ Links ]
Thomas, D.W., 1984, 'Vegetation in the montane forest of Cameroon', in S.N. Stuart (ed.), Conservation of Cameroon Montane Forest, pp. 20-27, International Council for Bird Preservation, Cambridge. [ Links ]
Thomas, D.W., 1996, Botanical Survey of the Rumpi Hills and Nta Ali with Special Focus on the Submontane Zone above 1,000 m Elevation, Final report to German Technical Service, Korup Project, Mundemba, Cameroon, p. 95. [ Links ]
Thomas, D.W., 1997, Botanical Inventory of Ejagham Forest Reserve Cameroon, Final report to Korup Project, Mundemba, Cameroon, p. 92. [ Links ]
Thomas, D.W. & Cheek, M., 1992, Vegetation and plant species in the proposed Etinde Reserve, Report to Government of Cameroon from Overseas Development Authority, Royal Botanic Gardens, Kew, London, UK, p. 43. [ Links ]
Thomas, D.W. & Thomas, J., 1996, Tchabal Mbabo botanical survey, World Wide Fund for Nature (WWF), Yaounde, Cameroon, p. 90. [ Links ]
Thomas, D.W., Kenfack, D., Chuyong, G.B., Sainge, M.N., Losos, E., Condit, R.S. et al., 2003, Tree Species of South Western Cameroon: Tree distribution maps, diameter tables and species documentation of the 50-hectare Korup Forest dynamics plot, Center for Tropical Forest Science of the Smithsonian Tropical Research Institute, Washington, DC, p. 247. [ Links ]
Vallèrié, M., 1971, Notice Explicative No 45. Carte Pédologique du Cameroun Occidental à 1/1.000.00, Office de la Recherché Scientifique et Technique Outré-mer, Paris, p. 59. [ Links ]
White, F., 1983, Vegetation Map of Africa, United Nation Educational, Scientific and Cultural Organization (UNESCO), Natural Resources Research, Paris, 20, p. 356. [ Links ]
Zhong, L., Chang-Yang, C., Lu, P., Gu, X., Lei, Z., Cai, Y. et al., 2015, 'Community structure and species composition of the secondary evergreen broad-leaved forest: The analyses for a 9 ha forest dynamics plot in Wuyanling Nature Reserve, Zhejiang Province, East China', Biodiversity Science 23, 619-629. https://doi.org/10.17520/biods.2015110 [ Links ]
 Correspondence:
Correspondence:
Felix Nchu
felixnchu@gmail.com
Received: 09 June 2017
Accepted: 23 July 2018
Published: 08 Jan. 2019