Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.47 n.2 Pretoria 2017
http://dx.doi.org/10.4102/abc.v47i2.2124
ORIGINAL RESEARCH
Fungi and invasions in South Africa
Alan R. Wood
Weeds Division, ARC-Plant Protection Research Institute, South Africa
ABSTRACT
BACKGROUND: Fungi are a major component of the functioning of all terrestrial ecosystems.
OBJECTIVES: To increase awareness of fungi as drivers of ecosystem processes, including invasion biology.
METHOD: Here, I reviewed the information available regarding fungal invasions of native ecosystems in South Africa in the context of the National Status Report on Biological Invasions.
RESULTS: Only seven fungal species are regulated as invaders (all category 1b) under the National Environmental Management: Biodiversity Act (NEM:BA) A&IS regulations. Four of these species are not yet known to occur in South Africa. Similarly, under the NEM:BA A&IS regulations, two of the four species listed as prohibited (i.e. not present in the country but which would pose a threat if introduced) are already present in the country.
The actual number of alien fungi in South Africa is much greater. A preliminary listing of alien fungal species is made, with a total of 9 pathogenic species known to attack indigenous plants, 11 saprotrophic species, 1 fish pathogen, 23 host-specific pathogens of listed alien terrestrial plants, 61 ectomycorrhizal species and 7 host-specific pathogens deliberately introduced as biological control agents. The majority of fungal species were introduced to South Africa most likely via the introduction of crop plants as passengers, although there are as yet very little details available on pathways of introduction into South Africa.
CONCLUSION: For almost all aspects considered, it is concluded that there is simply not sufficient data to begin to understand the role and impact of fungal invasions in South Africa.
Introduction
The fungi are a highly diverse eukaryotic kingdom, with approximately 100 000 known species. Estimates of the likely total number of species vary widely, largely due to there being less information available for them in contrast to the relatively well known plant and animal kingdoms. An earlier widely accepted conservative estimate was 1.5 million species (Hawksworth 1991, 2001), although this was revised upwards with time to 5.1 million species (Blackwell 2011). The advent of high-throughput sequencing technology has allowed a more accurate estimation than previously, with an estimate based on soil fungi from 365 localities across the world suggesting fungal diversity would be within the range of 2-3.4 million species (Tedersoo et al. 2014). Thus, the number of described species represents between 2% and 6.7% of the estimated total fungal diversity. Although there are insufficient data available to give an accurate estimate for the number of fungi indigenous to South Africa, plant to fungi ratios generated by international studies would suggest there would be at least 171 500 indigenous species (Crous et al. 2006). In addition, there are a number of groups that were traditionally considered as fungi but are now accepted as belonging to the Amoebozoa (e.g. slime moulds) and Stramenopiles (e.g. water moulds, including downy mildews and Phytophthora).
Fungi are important components of every terrestrial ecosystem, as decomposers, food sources, pathogens or mutualists. Despite this importance, it is only recently that a growing body of published research is documenting how important fungi are as drivers of ecosystem function. Fungi, including organisms traditionally considered as fungi (hereafter included in the general term 'fungi'), are poorly known both in terms of their biodiversity and their ecology relative to what is known about plants and animals (Desprez-Loustau et al. 2007). Likewise, fungi have been little considered in invasion biology, despite numerous international invasions by fungal pathogens causing devastating economic and ecological damage (Fisher et al. 2012; Loo 2009). In addition to increased research on the threat posed to indigenous organisms (plants, animals and microbes) by invasive fungal pathogens, there is also a need for research on the ecological impact of invasive saprobic and mutualist fungi, and on the impact of other invasive organisms on indigenous fungi and the ecological services they provide (Desprez-Loustau et al. 2007; Litchman 2010). Many fungi grow and reproduce rapidly, traits associated with invasion by a range of organisms. An extreme demonstration of their ability to produce large numbers of spores is the 2.175 × 109 spores day-1 cm-2 of exposed spore-producing surface recorded for the bracket fungus Trametes pubescens (Schumach.) Pilát (Rockett & Kramer 1974). Intercontinental natural dispersal of spores by wind is well known in fungi, which led to a widespread belief that fungi were largely cosmopolitan, their distribution only limited by the availability of suitable environmental conditions. However, there is now an appreciation that many fungi are endemic to particular areas (Desprez-Loustau et al. 2007; Litchman 2010), and that many have been transported around the world by humans and have invaded new geographic ranges (Desprez-Loustau et al. 2007; Fisher et al. 2012; Litchman 2010; Loo 2009; Roy et al. 2014; Wingfield et al. 2001).
A number of reviews from an international perspective have been published on fungal invasions (Desprez-Loustau 2007; Fisher et al. 2012; Litchman 2010; Loo 2009), mutualists and plant invasion (Nuñez & Dickie 2014; Richardson et al. 2000), population biology (Gladieux et al. 2015) and soil mycobiota and plant invasion (Dawson 2015; Dawson & Schrama 2016; Reinhart & Callaway 2006). This review focusses on what is currently known about fungi and invasion in South Africa. In particular, invasion as relevant to the National Environmental Management: Biodiversity Act (Act No. 10 of 2004) (NEM:BA). A simple matrix illustrates various possible interactions between alien and indigenous fungi with alien and indigenous plants (or ecological communities) (Figure 1). This review is organised in a manner reflecting this matrix.
In South Africa, pathogens of crop plants have been well documented (Crous, Phillips & Baxter 2000; Doidge & Bottomley 1931; Doidge et al. 1953; Gorter 1977); however, they have not received attention as invaders of natural ecosystems. Doidge (1950) noted that with the introduction of crop plants into South Africa, there have been many introductions of pathogens of these crop plants, and that many were widespread and occurred wherever their hosts were cultivated. Indications are that the rate of introductions is increasing (Wingfield et al. 2001). These pathogens may potentially infect indigenous plants and invade natural ecosystems, yet it is only in the last few years that this potential threat is beginning to be assessed (e.g. Adams, Roux & Wingfield 2006; Cruywagen et al. 2016; Mehl et al. 2016). Many other non-pathogenic fungi have also been introduced, but no consideration has been given to these as invaders or to any potential negative impacts on indigenous ecosystems.
For the purpose of this review, the large literature on pathogens of crop and plantation plants in South Africa is not considered unless these fungi are known to affect indigenous biodiversity. This in no way implies that fungal pathogens of crop plants are not important, but control of these disease-causing organisms falls under the jurisdiction of the Plant Health and Animal Health directorates of the Department of Agriculture, Forestry and Fisheries (DAFF) and is regulated by the Agricultural Pests Act, (Act No. 36 of 1983). There is a need to investigate the spill over of introduced crop pathogens into natural ecosystems. Aquatic and marine fungi, and fungi introduced for food production, are not considered in this review. Despite an increasing awareness of invasive fungal diseases of vertebrate wildlife (Allender et al. 2015; Berger et al. 2016; Tomkins et al. 2015), only one report from South Africa was found, namely a disease-causing water mould, Aphanomyces invadans Willoughby, Roberts & Chinabut, causal agent of mycotic granulomatosis in fish. This has been found in widespread localities in southern Africa, including the Western Cape province (Huchzermeyer & Van der Waal 2012). Fungal pathogens of vertebrates (including humans) are also not further considered in this review, although there is a clear need for vigil against potential invaders and studies on species already present.
Diversity of indigenous fungi
Knowledge of indigenous biodiversity is fundamental to being able to evaluate whether an organism is indigenous or alien (Desprez-Loustau et al. 2007). In South Africa, there has been a long tradition of documenting indigenous fungi, starting with European plant collectors who explored South Africa such as Carl Peter Thunberg (1743-1828) and William John Burchell (1781-1863) (Doidge 1950; Rong & Baxter 2006). The National Collection of Fungi (PREM) houses less than 70 000 specimens, which includes all historical collections that were previously housed in other herbaria in the country, as well as specimens from other continents, although much of the early collections (including type specimens) are housed in various European herbaria (Rong & Baxter 2006). Doidge (1950) remains the only comprehensive listing of fungi recorded from southern Africa. Many of the listed fungi have so far only been recorded as pathogens of introduced crop plants. A total of 4748 species were listed (Doidge 1950), only a small fraction of the total expected (∼171 500, Crous et al. 2006). Even with an increase in the rate of species described from South Africa since 1950, particularly since the advent of molecular phylogenetic techniques, the number of named indigenous species is only a small fraction (∼3%) of the likely total diversity.
Indigenous fungi remain little documented, other than certain groups of plant pathogens, macrofungi and lichens. For example, arbuscular mycorrhizae (AM) are common mutualists of South African plants (Allsopp & Stock 1993; Gaur et al. 1999; Hawley & Dames 2004; Straker, Weiersbye & Witkowski 2007) and are considered essential to ecosystem functioning (van der Heijden et al. 2015). To date only five species have been named from indigenous plants in South Africa (Blaszkowski et al. 2010; Gaur et al. 1999), and a further four species have been recorded from cassava (Straker, Hilditch & Rey 2010). In contrast, a total of 43 species have been recorded from semi-arid regions in Namibia (Stutz et al. 2000; Uhlmann et al. 2004) and 147 virtual taxa were obtained by 454 pyrosequencing from Gorongosa National Park, Mozambique (Rodriguez-Echeverria et al. 2016), suggesting that most of the indigenous biodiversity of this group of fungi is yet to be documented in South Africa. Many such examples from a wide variety of major taxa could be cited.
It can be concluded that, despite efforts by the few mycologists who have operated in South Africa, baseline biodiversity information is too little to be of any use in determining the alien or indigenous status of fungi newly discovered in South Africa, except in some specific cases.
Alien fungi
The human-assisted distribution of fungi around the world has been, and remains, the most important pathway of introduction (Anderson et al. 2004; Desprez-Loustau et al. 2007; Fisher et al. 2012; Roy et al. 2014; Wingfield et al. 2001). The trade in live plants is an important route of introduction to, and further spread within, continents and countries (e.g. Carnegie & Cooper 2011; Moralejo et al. 2009; Roy et al. 2014). Although there is a general trend amongst pests and pathogens of crop plants introduced around the world that dispersed range increases with wider host range, introduced fungi have the narrowest host range but the widest introduced range (Bebber, Holmes & Gurr 2014), suggesting that many fungus species are well adapted for human-assisted dispersal and invasion. Propagule characteristics related to successful long-distance dispersal, allowing for rapid spread once introduced, were good predictors of invasion success by fungi (Philibert et al. 2011). As a result of several hundred years of introducing horticultural, crop and plantation plants, and plant products, it is likely that large numbers of fungi have been introduced to South Africa.
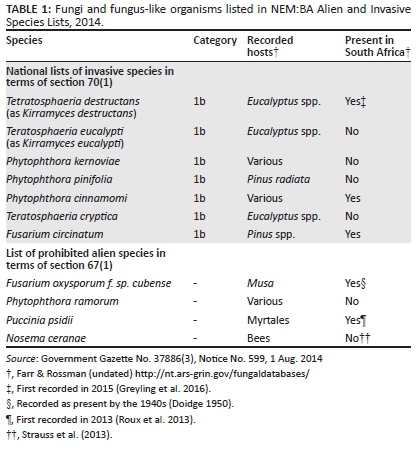
There is no literature available specifically on fungi and natural ecosystem invasions in South Africa. The first attempt at listing invasive fungi was published in the NEM:BA A&IS regulations (Notice No. 599, 1 Aug. 2014), which lists a total of seven fungi (list 11 of the regulations), all placed in category 1b (Table 1). Of these, three are recorded only from species of Eucalyptus, and two are recorded only from Pinus species. In addition, four of these species have not yet been recorded in South Africa (Farr & Rossman undated). Thus, this list does not in any way reflect alien microbial species important in natural ecosystems in South Africa. Only one of the listed species is well known as a pathogen of indigenous plants (Phytophthora cinnamomi Rands; Lübbe & Mostert 1991; Nesamari, Coutinho & Roux 2016; Von Broembsen 1984a; Von Broembsen & Kruger 1985). Rather this reflects the reality that there is not a single researcher employed in South Africa with the specific mandate to undertake studies on alien fungi in natural ecosystems, nor has there been one. What is known is scattered piecemeal in the mycological and plant pathological literature. An attempt is made here to start to bring some of this literature together. Information provided here can be used as a springboard to compile future lists that take into account both invasive status and whether a National Management Programme could be developed and implemented for invasive species, as required by the legislation for each listed organism.
How can fungi be recognised as alien? In general, host-specific pathogenic or mutualistic fungi can be recognised as alien when their hosts are themselves alien. However, few fungal species that can be recognised as introduced have been assessed as to whether they attack indigenous plants or invade natural ecosystems. For example, the polypore Laetiporus sulphureus (Bull.) Murrill is well known as a pathogen of various Northern Hemisphere trees in their native and introduced ranges, but it has also been recorded on the native Olea europaea L. ssp. africana (Mill.) P.S. Green in South Africa (Doidge 1950). This old record suggests that it might also damage native trees, but this requires confirmation. Non-specific pathogens, saprotrophs, endophytes and mutualistic fungi are more difficult to assess as alien. Morphologically characteristic macrofungi could be identified as being alien if they were not recorded in the early South African literature. For example, the Australian Aseroë rubra Labill. was only confirmed as present in South Africa since the 1950s (Coetzee 2010). Likewise, the European Coprinopsis picacea (Bull.) Redhead, Vilgalys & Moncalvo, was not listed by Doidge (1950) or Pearson (1950) but has recently been reported on the i-spot website (http://www.ispotnature.org/communities/southern-africa). Detailed molecular analyses comparing genetic diversity within South African isolates, or comparing local genotypes to overseas ones, will be necessary to identify most alien species. The European Armillaria mellea (Vahl) P. Kumm. and Armillaria gallica Marxm. & Romagn. were identified as introduced by such detailed studies (Coetzee et al. 2001, 2003). Therefore, only a few fungi can be singled out which are definitely alien and have naturalised in South Africa (Table 2), and even fewer have been recognised as having a detrimental impact on our natural environments.
Fungi and theories of invasion biology
Many hypotheses have been put forward to explain why and how species become invasive after introduction to a region where they are not native (Catford, Jansson & Nilsson 2009). Fungi are considered to have positive or negative impacts on invasive plants in various of these hypotheses (Catford et al. 2009), although as yet there has been no consideration of which of the hypotheses are relevant directly to fungi as invaders.
Recently there has been an interest in the interaction of soil biota, including fungi other than mycorrhizas, and invasive plants. Soil microbiota may have a direct positive (Cui & He 2009; Gundale et al. 2014), negative (Lankau 2010; Vitullo et al. 2014) or no impact (Bennett & Strauss 2013; Birnbaum & Leishman 2013) on the growth and invasiveness of alien plants. However, these interactions are dependent on many variables including the ecologically functional groups of the microorganisms considered, as well as the surrounding vegetation and interactions with other organisms (Reinhart & Callaway 2006). Phylogenetically close plant relatives may produce a selective pressure resulting in similar soil microbiota on their roots (Wehner et al. 2014), which can facilitate invasion by alien close relatives more than the microbiota associated with non-relatives (Hill & Kotanen 2012; Reinhart & Callaway 2006). Indirect interactions also occur, a positive soil microbe-plant feedback has been shown to increase growth of Impatiens glandulifera Royle and increase foliar endophyte fungus levels that may provide enhanced protection from herbivores (Pattison et al. 2016). In general, native plants grow better in 'home' soil whereas invasive plants are more catholic and less affected by the soil source (i.e. derived from below native or invasive plants). Overcoming the native plants' 'home' soil advantage by invasive plants is dependent on exceeding a threshold of abundance, external disturbances or having a competitive or dispersal advantage relative to the native plants (Suding et al. 2013).
Invasive plants alter the soil microbiota composition (Elgersma & Ehrenfeld 2011), altering abiotic soil properties such as pH and nutrient levels (Duchicela et al. 2012; Kourtev, Ehrenfeld & Häggblom 2003). In turn, these altered soil conditions affect indigenous plants negatively, although the level of impact is species specific (Scharfy et al. 2010).
Differences in above-ground and below-ground fungal pathogens occur between the native and invaded range of alien plants. The Enemy Release hypothesis suggests that plants succeed as invaders because they have been introduced without their herbivores and pathogens. The practice of classical biological control, the introduction of co-evolved host-specific pathogens (or other organisms), is based on this hypothesis. Release from soil pathogens (Reinhart et al. 2010; Van Grunsven et al. 2009), as well as from generalist foliar and seed pathogens (Halbritter et al. 2012), have also been reported as important factors in the invasion by introduced plants. However, enemy escape also occurs on a local scale in the native range of invasive plants (MacKay & Kotanen 2008) and is not correlated with invasiveness of many plants (van Kleunen & Fischer 2009). Also, escape from one functional guild of pathogens can be counteracted by non-escape from other guilds (Agrawal et al. 2005), and the accumulation of pathogens native to the invaded range which damage the invasive plant may counter benefits derived from the initial enemy release (Flory & Clay 2013; Flory, Kleczewski & Clay 2011; Vitullo et al. 2014). Native pathogens that limit or reduce initial invasion are considered to be a Biotic Resistance mechanism (Catford et al. 2009). Contrary to this last premise, the Accumulation of Local Pathogens hypothesis suggests that if the invasive plant is less susceptible than native plants, accumulating high levels of local generalist pathogens increases invasion success by suppressing native plant growth (Eppinga et al. 2006; Mangla, Inderjit & Callaway 2008). Dawson (2015) and Dawson and Schrama (2016) provide a theoretical framework incorporating these hypotheses and plant-soil feedbacks to understand how changes in soil mycobiota facilitate invasion by plants.
Plant-soil microbe interactions or invasion processes may apply inconsistently to individual plant species across their invasive range or change with time since invasion. In California (USA), Ammophila arenaria (L.) Link accumulated local pathogens suppressing native plants (Eppinga et al. 2006), whereas in South Africa this was not the case. Rather, Biotic Resistance associated with native grasses was an important determinant of where this plant was more or less invasive (Knevel et al. 2004). Release from the soil pathogens in its native range, South Africa - Enemy Release - has been demonstrated to be important to Carpobrotus edulis (L.) L. Bolus invasion of the Mediterranean (Van Grunsven et al. 2009) or not (de la Peña et al. 2010). In the latter study, Biotic Resistance was shown to be an important initial factor but with time this was overcome and the plant modified the soil microbiota in its favour. Native fungi accumulated on roots of Vincetoxicum rossicum (Kleopow) Pobed. have variously been shown to increase its growth or not, and to have negative impacts on only some native plants (Day, Dunfield & Antunes 2015, 2016).
Modifying the soil biota to favour the invader plant and be deleterious to native plants may also produce phenotypic changes in the invader. Ageratina adenophora Spreng. modifies the soil microbiota in China, by reducing AM fungi and increasing the ratio of bacteria to fungi to the detriment of native plants (Niu et al. 2007; Xingjun et al. 2005). In addition, plants in the China had higher leaf nitrogen levels and surface area compared with plants from the native range (Feng et al. 2011). The accumulation of native Fusarium root pathogens benefitted Chromolaena odorata (L.) R.M. King and H. Rob. in India (Mangla et al. 2008) but not in South Africa (te Beest et al. 2009). However, in the latter study, plants increased stem biomass and height growth when grown in unsterilised soil from the invaded range (te Beest et al. 2009). The above few studies of fungal interactions with alien plants in South Africa need to be expanded, and other invasive plants included, to determine whether there are any generalities of invasion mechanisms peculiar to South Africa. Comparing mechanisms of invasion in South Africa with that occurring elsewhere in the world would provide useful information; for instance, does A. ageratina also benefit from modifying the soil microbiota here as it does in China? And why does soil biota affect the invasion of A. arenaria and C. odorata differently in South Africa compared with other parts of the world?
Alien fungi and alien plants
Host-specific pathogens
Amongst the numerous pathogenic fungi accidentally introduced along with their alien host plants (including crop, plantation, horticultural and accidentally introduced invasive species) are species that attack invasive plants. Some of these, primarily host-specific pathogens, may well help to suppress the populations of their hosts, providing a free biological control service (Table 3). The list provided is not complete, as the plants listed are primarily declared weeds (NEM:BA A&IS) and could be greatly expanded if other alien plants are included (e.g. Uromyces bidenticola Arthur and Entyloma bidentis Henn. are both common on Bidens pilosa L.).
The deliberate introduction of alien fungi as biological control agents of their co-evolved host plants began in South Africa with the release of Uromycladium tepperianum (Sacc.) McAlpine against Acacia saligna (Labill.) Wendl. in 1987. To date, a total of nine fungi have been approved for release, permission for release of two was obtained in late 2015 and efforts are currently underway to release these in the field. Of the other fungi released, five established well whereas two failed to establish. Two of the established fungi have a considerable impact on their host's population densities (U. tepperianum, Entyloma ageratinae R.W. Barreto & H.C. Evans). Reviews of biological control of invasive alien plants using plant pathogens in South Africa have been published (Morris 1991; Morris, Wood & den Breeÿen 1999). See Zachariades et al. (2017) for a recent review of the effectiveness of biological control in general in South Africa.
Ectomycorrhizal fungi
The role of mycorrhizas in plant invasions has recently been reviewed (Nuñez & Dickie 2014; Richardson et al. 2000). The majority of invasive plants in South Africa are AM or ectomycorrhizal (EM); some invasive plants are however non-mycorrhizal (e.g. Hakea species) (Richardson et al. 2000). Pinus and Eucalyptus are both obligatory EM plant genera, and a large number of EM fungi have been introduced into South Africa, either deliberately or accidentally (Vellinga, Wolfe & Pringle 2009), allowing the widespread cultivation of these plantation plants. Vellinga et al. (2009) listed 45 species from South Africa, although an additional 17 species have also been recorded (e.g. Hawley, Taylor & Dames 2008; Martin et al. 2002; van der Westhuizen & Eicker 1994) (Table 4), and therefore the total number of introduced EM fungi may be more than currently known.
Pines are recognised as being important invaders in mountain catchment areas in South Africa. This invasion is a dual invasion by the plants, which are obligately EM and their associated EM fungi (Collier & Bidartondo 2009; Dickie et al. 2010; Hynson et al. 2013). Initially, the fungal inoculum available is low, slowing the invasion of the plants. However, with time, the pines and their EM fungi proliferate and replace the indigenous vegetation and their mycorrhizal fungi (Collier & Bidartondo 2009). Currently, there have been no studies investigating which EM fungi are co-invading South Africa's natural ecosystems; this may be a small subset of the total diversity of introduced species (Dickie et al. 2010; Hynson et al. 2013).
It has been found that usually the EM fungal communities on alien plantation trees consist of introduced species normally associated with those plants in their native habitats, even when indigenous EM fungi are present in the surrounding natural ecosystems (Dickie et al. 2010; Jairus et al. 2011). Yet, several EM fungi have been recorded as invading natural ecosystems, including Amanita muscaria (L.) Lam. and Amanita phalloides (Vaill. ex Fr.) Link (Dunk, Lebel & Keane 2012; Pringle et al. 2009). The presence of indigenous EM fungi and host plants potentially allow for host shifts by introduced EM fungi and invasion of natural ecosystems, given time to adapt to local conditions (Jairus et al. 2011).
Approximately 400 species of indigenous putatively EM fungi have been reported from tropical Africa (Verbeeken & Buyck 2002). In South Africa, the only indigenous fungi known to be EM symbionts are the desert truffles (Kalaharituber pfeilii (Henn.) Trappe & Kagan-Zur, Eremiomyces echinulatus (Trappe & Marasas) Trappe & Kagan-Zur, Mattirolomyces austroafricanus (Marasas & Trappe) Kovács, Trappe & Claridge) (Trappe et al. 2008). Introduced EM fungi likely pose little direct threat to natural ecosystems in South Africa, although north of South Africa's borders there is a potential threat. Controlling invading pines, or other EM plants, would control any invasion by their associated EM fungi. However, the co-invasion of EM plants and associated EM fungi can reduce indigenous mycorrhizal fungi (Becklin, Pallo & Galen 2012). Once reduced or lost, it may take many years for the indigenous AM fungi to recover (Lankau et al. 2014), reducing the ability of AM plants to recover post control of the invaders. Many fynbos plants are associated with AM fungi (Allsopp & Stock 1993). In a study conducted in South Africa, a single cycle of invasion (by ectomycorrhizal Pinus pinaster Aiton and the non-mycorrhizal Hakea sericea Schrad. & J.C.Wendl.) did not reduce the development of AM fungi in indigenous plants following clearing, likely due to the persistence of AM plants at low densities within the invaded area during the invaded period (Allsopp & Holmes 2001). However, the impact of successive cycles of invasion has not been studied. The impact would likely be greatest on ericoid mycorrhizae as these are very slow to re-establish (Allsopp & Holmes 2001).
Alien fungi and indigenous plants
Non-host specific pathogens
Amongst the many fungi introduced to South Africa are those which are not host specific, but with a broader host range they may potentially attack indigenous plants, either closely related to their natural host or to a wide range of phylogenetically unrelated plants. Internationally, severe ecological impacts have been recorded by a wide range of fungal pathogens (Desprez-Loustau et al. 2007; Fisher et al. 2012). P. cinnamomi is one of the most serious invasive alien pathogens of natural ecosystems, as well as a pathogen of numerous crop species, around the world including in South Africa. It is the causal organism of Jarrah Dieback in Western Australia, killing a broad range of plants. Widespread in Australia, it kills many plant species in various ecosystems (Cahill et al. 2008) and is also associated with disease of naturally growing trees in other parts of the world (e.g. Balci et al. 2007; Camilo-Alves, Clara & Ribeiro 2013; Vettraino et al. 2005). In South Africa, it is the causal organism of a root and crown rot of Leucadendron argenteum (L.) R. Br. (van Wyk 1973), dieback of Ocotea bullata (Burch.) E. Meyer (Lübbe & Mostert 1991) and recently has been found to have caused some mortality of Encephalartos transvenosus Stapf & Burtt Davy (Nesamari et al. 2016). It has been recorded as causing mortality of a wide range of indigenous fynbos plants, especially members of the Proteaceae (Von Broembsen 1984a; Von Broembsen & Brits 1985; Von Broembsen & Kruger 1985), including in remote pristine mountain fynbos (Von Broembsen 1984b) and has been found in headwaters of rivers in the fynbos biome (Von Broembsen 1984b). Low genetic diversity in South African populations indicates that it is an introduced species (Linde, Drenth & Wingfield 1999).
Several other species of Phytophthora are likely invasive in South Africa. Phytophthora multivora P.M. Scott & T. Jung and P. capensis Bezuid, Denman, A. McLeod & S.A. Kirk have been isolated from several indigenous plants (Bezuidenhout et al. 2010). The origin of these species is not known, but they may well be aliens. Phytophthora niederhauserii Z.G. Abad & J.A. Abad is a recently described species known to infect grapevines in South Africa. Considering that it has been recorded from a wide variety of plant species worldwide (Abad et al. 2014), it is likely to also be invasive in South Africa. Other species known so far only as crop pathogens in South Africa may also be invasive. The related genus Pythium also contains some species which have been isolated from indigenous plants or associated soil (McLeod et al. 2009) including P. irregulare Buisman isolated from Aspalathus liearis (Burm.f.) R. Dahlgren in natural ecosystems (Bahramisharif et al. 2014). An unidentified Pythium species has been associated with dieback and mortality of fynbos shrubs in the Western Cape (Jacobsen et al. 2012).
Puccinia lagenophorae Cooke is a rust fungus originating in Australia and which has since been introduced around the world. It was present in South Africa by 1918, and has been recorded on 48 indigenous species in the Asteraceae, and is now distributed from the Cape Peninsula to the Richtersveld and Mpumalanga (Scholler et al. 2011). It has also been recorded as having formed a hybrid with an unknown, presumably indigenous, species in KwaZulu-Natal (Morin et al. 2009); hybridisation is an important means of speciation amongst fungi leading to the emergence of new invasive species (Brasier 2001; Stukenbrock 2016).
The myrtle rust Puccinia psidii G. Winter, originally from South America, has been introduced around the world in Eucalyptus plantations and has caused significant damage to indigenous Myrtales plants in Australia (Carnegie et al. 2016; Pegg et al. 2014). This fungus is listed as a prohibited species in the NEM:BA:A&IS regulations but has recently been recorded as present in South Africa (Roux et al. 2013). A risk assessment of selected indigenous species in the Myrtales found that this rust fungus does pose a threat to our indigenous flora (Roux et al. 2015). This fungus has now been found to be widespread in South Africa, ranging from southern KwaZulu-Natal to Limpopo provinces, and occurring on a range of introduced and indigenous plants in the Myrtales, but so far not on Eucalyptus. The genotype found in South Africa is distinct to that which is invasive elsewhere in the world (Roux et al. 2016).
A. mellea was introduced to the Company Garden, Cape Town, possibly as long as 300 years ago (Coetzee et al. 2001). It has, until recently, been limited to this small area by the development of the City of Cape Town isolating this locality, but has now been recorded from Kirstenbosch on indigenous Proteaceae (Coetzee et al. 2003; Wingfield et al. 2010). Likewise, Armillaria gallica has also been recorded from Kirstenbosch (Coetzee et al. 2003). Both these fungi are from the Northern Hemisphere and are highly destructive wood-rot pathogens of a wide range of tree species. They may well pose a significant threat to indigenous forests if they establish therein.
The endemic and endangered wild rye (Secale strictum C. Presl ssp. africanum (Stapf) K. Hammer) is susceptible to, and infected by, Puccinia graminis Pers., P. striiformis Westend. and P. recondita Dietel & Holw., all introduced and widespread pathogens of cereal crops in South Africa. Infection by these rust fungi may have contributed to the almost complete loss of this grass, now restricted to a single farm on the Roggeveld (Pretorius, Bender & Visser 2015).
Ceratocystis pirilliformis I. Barnes & M.J. Wingf., an introduced fungus which colonises wounds on Eucalyptus (Nkuekam et al. 2009), has recently been found on Rapanea melanophloeos (L.) Mez, and it is suggested that ongoing distribution of this pathogen is by anthropogenic activities (Lee et al. 2016).
There are likely many more alien fungi attacking indigenous plants. Non-specific pathogens introduced with alien host plants can be more damaging to native plants than to the original host, facilitating invasion by the alien plant (Li et al. 2014). However, recognition of these as invaders is difficult, and there have been few studies till recently looking for alien fungi in natural systems in South Africa. Erythricium salmonicolor (Berk. & Broome) Burds. has been recorded as causing disease and even death of indigenous trees such as Podocarpus spp. and Dais cotonifolia L. near to plantations of Eucalyptus and Acacia mearnsii De Wild.. However, it was not conclusively determined if this was an alien pathogen introduced in association with the plantation industry, or indigenous (Roux & Coetzee 2005). It is likely alien. There is a need to investigate many pathogens of commercial and plantation crops as potential invaders of natural habitats in South Africa. The Botryosphaeriaceae illustrate this point; they are a highly diverse family of woody plant inhabiting fungi that may be endophytes or latent pathogens, the latter only causing disease under conditions of environmental stress on the host plant (Slippers & Wingfield 2007). Many species have been recorded from introduced crop plants and indigenous plants such as members of the Proteaceae (Marincowitz et al. 2008), Syzygium cordatum Hochst. ex Krauss (Pavlic et al. 2007), Adansonia digitata L. (Cruywagen et al. 2016) and Sclerocarya birrea subsp. caffra (Sond.) Kokwaro (Mehl et al. 2016) in South Africa. Species recorded from indigenous plants include wide spread species recorded from many host plants, whereas there is also a high diversity of indigenous members of this family. There is a need to determine which are alien and which may cause disease in indigenous plants of sufficient severity to impact these plants' population dynamics, if any.
Saprotrophic fungi
Pearson (1950:277) noted that the identification of species of mushrooms collected in and around Cape Town proved to be easy as 'Most of the agarics and all the boleti collected are the same as found in Europe'. This was ascribed to most trees being of foreign origin, and few mushrooms occurring in natural forest and fynbos (Pearson 1950). It is therefore likely that many saprophytic macro- and microfungi are introduced and naturalised. A high proportion of the macrofungi named to species level in South Africa have been recognised because they are widespread European or Northern Hemisphere species (e.g. Gryzenhout 2010; van der Westhuizen & Eicker 1994). An example is the morel Morchella esculenta (L.) Pers. (Doidge 1950; Gryzenhout 2010); this genus is recognised as indigenous to the Northern Hemisphere (O'Donnell et al. 2011). Because macrofungi are relatively well known throughout much of the developed world, the majority of saprophytic fungi that are recognised as naturalised are these rather than microfungi. In France, 26% (59 of 227 species) of fungi recorded as alien were saprotrophs; all were macrofungi, and all but one species belonged to the Agaricales, Polyporales and Phallales (Basidiomycota) (Desprez-Loustau et al. 2010).
Many naturalised alien saprotrophs are, however, likely to simply occupy ecological space rather than damaging natural ecosystem functions (Vacher et al. 2010). Favolaschia calocera R. Heim has been recorded as having invaded many countries around the world but is not considered damaging to the environment anywhere (Vizzini, Zotti & Mello 2009). However, this requires testing as these fungi have not been investigated as to whether they potentially disrupt or change ecosystem processes, or replace indigenous species.
Indigenous fungi and alien plants
A number of fungi regarded as indigenous to South Africa cause diseases of plantation trees which are also invasive alien plants, including Ceratocystis albifundus M.J. Wingf., De Beer & M.J. Morris (wilt disease of Acacia mearnsii) (Roux et al. 2007) and Chrysoporthe austroafricana Gryzenh. & M.J. Wingf. (canker disease of Eucalyptus) (Heath et al. 2006). Several related fungi described from indigenous trees have proven to be pathogenic to plantation trees in inoculation experiments (Kamgan et al. 2008; Nakabonge et al. 2006; Pavlic et al. 2007). Various other fungi likely to be indigenous to South Africa have been recorded on naturalised weeds or invasive plants (Table 3).
These new associations allow the deliberate use of these pathogens as biological control agents. Two fungi have been developed as biocontrol agents of alien plants in South Africa: Colletotrichum acutatum J.H. Simmonds which causes gummosis disease of H. sericea (Morris 1983, 1989) and the wood-rotting Cylindrobasidium laeve (Pers.) Chamuris which is used to prevent coppicing of felled Acacia mearnsii trees (Morris et al. 1999). Another is under development, Pseudolagarobasidium acaciicola Ginns, which causes a dieback disease of Acacia cyclops G. Don (Kotze, Wood & Lennox 2015; Wood & Ginns 2006). More could be developed.
The high biomass or seed production of invasive plants may lead to high levels of associated generalist local pathogens, not sufficiently damaging to the invader's population dynamics to exert control, but which can spill over onto indigenous plants and can reduce the native populations (Beckstead et al. 2010). Accumulation of local pathogens can also provide a mechanism promoting invasion (see above). Accumulation of local pathogens can persist even after the removal of the alien plants, leaving a legacy of increased damage to recovering indigenous vegetation (Maoela et al. 2016).
Arbuscular mycorrhizae
AM fungi are widespread in terrestrial ecosystems and are considered to be non-specific, and therefore available to invasive plants to form symbiotic associations (Redecker & Raab 2006; Richardson et al. 2000). Although able to infect most plants, AM fungi can show host preferences, thus the interaction between invasive plants and the available AM fungi may result in a positive feedback which changes the composition of the AM community to the benefit of invasive plants and to the detriment of indigenous plants (Zhang et al. 2010). Various impacts of AM have been recorded facilitating invasion by alien plants. A. saligna (Labill.) Wendl. has a larger root system than indigenous Fabaceae of the fynbos biome, and therefore has more extensive AM fungal colonisation providing more efficient nutrient uptake which promotes invasion (Hoffman & Mitchell 1986). Disturbance can reduce the amount of AM fungus inoculum available, providing a window of opportunity for non-dependent invasive plants to establish dominance over AM-dependent native plants (Carvalho et al. 2010; Owen et al. 2013). Allelopathic invasive plants can also reduce AM fungi, resulting in reduced growth of native plants dependent on these fungi (Hale, Lapointe & Kalisz 2016; Koch et al. 2011), although this effect can be offset by soil microbiota, possibly by the degradation of the allelopathic chemicals (Lankau 2010).
Fungi and arthropods
Little work has been done on fungi associated with insects and other arthropods in South Africa. Rong & Grobbelaar (1998) listed records of fungi (excluding microsporidia) found associated with arthropods in South Africa. Amongst these are fungi that have potential to be developed as biological control agents of arthropod pests (Rong & Grobbelaar 1998). Several microorganisms have been developed internationally as commercial biopesticides, such as the fungi Beauveria bassiana (Bals.-Criv.) Vuill. and Metarhizium anisopliae (Metschn.) Sorokin, and the bacterium Bacillus thuringiensis Berliner. Several of these products are marketed in South Africa; however, no ecological risk assessment of using alien strains of these microorganisms, isolated and commercially produced on other continents, has been undertaken in South Africa. The development of indigenous isolates, adapted to the local environment, requires investigation. There are a few local companies developing these. Naturally existing entomopathogenic fungi can also be managed as part of an integrated pest management programme of introduced pests of crops (Hatting et al. 1999; Hatting, Poprawski & Miller 2000) and invasive alien insects.
Control measures
The author is not aware of any attempts in South Africa to control an invasive fungus in a natural ecosystem. Preventing the introduction of potentially ecologically damaging fungi is the best method of mitigating impacts (Dickie et al. 2016; Hansen 2008; Wingfield et al. 2001). Part of the quarantine process is to prohibit the introduction of named species considered to pose a risk to the country. The current list of prohibited species in the NEM:BA:A&IS regulations include four fungus species (Table 1), two of which already occur in the country. Fusarium oxysporum Schltdl. f. sp. cubense W.C. Snyder & H.N. Hansen, cause of Panama disease of bananas, was recorded as present in South Africa by the 1940s (Doidge 1950). Various pathogens can be recognised as likely posing a risk to native ecosystems, and need to be added to the list of prohibited species. An example is Armillaria luteobubalina Watling & Kile, an Australian species pathogenic to many plant species (e.g. Shearer et al. 1997,Shearer & Tippert 1988). This list must be compiled in cooperation with DAFF who administer their own list, and administer inspection and quarantine services. Phytophthora kernoviae Brasier, Beales & S.A. Kirk is better placed in the list of prohibited species rather than in the invasive species lists. A shortfall of such lists is that undescribed or little known but potentially devastating pathogens will not be listed.
If a damaging fungus is introduced and has become widespread, control measures that can be implemented are focused on (1) stopping further spread of the fungus, (2) reducing inoculum levels within the area affected and (3) restoring damaged vegetation (Hansen 2008). Early control methods relied on stopping further spread by restricting entry to infected areas and strict hygiene imposed on any person or vehicle entering infected areas, for Phytophthora species (Hansen et al. 2000; Shearer & Tippert 1988). These remain as important control measures, and may be the only practical measures that can be applied (Dickie et al. 2016). Identification of high hazard areas, where the fungus is most likely to invade and cause damage, focusses these control methods where most needed (Cahill et al. 2008; Shearer & Tippert 1988). Fungicides can be applied for control (Cahill et al. 2008; Dickie et al. 2016; Dunstan et al. 2010; Hardy, Barrett & Shearer 2001; Hill, Tippert & Shearer 1995); however, these can have negative impacts on native plants (phytotoxicity) (Hardy et al. 2001) or fungi (Dickie et al. 2016).
Eradication of pathogens that have a limited host range can be successful, following early detection of outbreaks in geographically limited areas, by eradication of host plants (Ganley & Bulman 2016; Sosnowski et al. 2009). This has been unsuccessful when applied in natural ecosystems to pathogens that have efficient long-distance dispersal mechanisms (e.g. P. psidii, Carnegie & Cooper 2011) or broad host ranges (e.g. P. cinnamomi, Hill et al. 1995). However, the combination of eradicating host plants, soil fumigation and installation of a physical root barrier proved to be successful in eradicating P. cinnamomi in a small area adjacent to a high-value conservation area. The topography at this site limited the spread of this pathogen by water-borne spores (Dunstan et al. 2010).
Conclusions and recommendations
The purpose of this review has been to provide a very brief summary of some of the information that has become available on fungi and invasions in the scientific literature, to provide a springboard for future investigations of fungal invasion in South Africa. The broad phylogenetic diversity and numerous ecological roles of fungi result in multiple interactions with other organisms. The best known ecological impacts by invasive fungi are of introduced tree pathogens that have caused significant reductions to their new-association host's abundance (Cahill et al. 2008; Fisher et al. 2012; Loo 2009). In South Africa, the most widespread and damaging is P. cinnamomi, although the impact locally in natural ecosystems has not yet been quantified. But, not all naturalised species cause ecological damage to natural ecosystem functioning. Many fungi, including pathogens, are assimilated into existing plant-fungus ecological networks (Vacher et al. 2010). Internationally, it is only relatively recently that an interest in the fungal dimension in invasion science has developed, with inventory, impact and invasion process studies beginning to be published. Apart from terrestrial plant pathogens, very little is still known about naturalised saprotrophs (Desprez-Loustau et al. 2007; Litchman 2010), plant mutualists, animal pathogens and aquatic fungi.
One consequence of invasions by fungi is the increased potential for hybridisation leading to either novel genotypes or even new species that may be more aggressive, or attack different hosts, than the parents (Brasier 2001; Callaghan & Guest 2015; Stukenbrock 2016). Fungi can rapidly adapt to new environments and novel hosts, and then these novel genotypes can rapidly disperse, facilitating invasion (Gladieux et al. 2015). Traits that have been associated with fungal invasions include (1) characteristics associated with long-distance dispersal; (2) sexual reproduction; (3) spore shape, size and number of cells; (4) pre-adaption to environmental conditions of introduced range; and (5) parasitic specialisation (Philibert et al. 2011). Fungi have been implicated in a number of hypotheses explaining invasion by plants but have not yet been investigated as to which are relevant to their invasions. In addition to direct impacts of invasion, fungi (and other microbes) interact in many different ways with other organisms within ecological networks, so that there are also many indirect impacts of fungi (both indigenous and alien) on invasions (Dawson & Schrama 2016; Desprez-Loustau et al. 2007).
In terms of the National Status Report as set out in the NEM:BA A&IS regulations (Government Notice No. R598, 1 August 2014), it can be concluded that the fungi listed as category 1b are mainly not damaging to natural ecosystems (five of the seven listed are plantation crop pathogens, four are not yet present in the country) and therefore irrelevant to the regulations, that no risk assessment of any fungus species as a potential invader has been undertaken and no control measures have been implemented by the Department of Environmental Affairs (DEA) against any listed species.
This situation exists because there is simply no information available to evaluate the invasive status of most fungi. Interventions required include the development of capacity to document the fungi indigenous to South Africa. This will also provide capacity to document invasive fungi, and record new arrivals. Capacity to investigate ecological processes driven by or influenced by fungi also needs to be developed, so that the impacts of invasive species can be determined, including by invasive mutualistic and saprotrophic fungi. This capacity would also be relevant to determine possible means of reducing impacts by invasive fungi, although there is limited scope for direct control. Fungi also have potential as biological control organisms, and efforts to develop agents against invasive alien plants should continue. There is under-exploited potential for the development of locally occurring agents against invasive or economically important pathogens (e.g. Trichoderma spp.), arthropods (e.g. B. bassiana) and other animal groups such as nematodes.
If an alien fungus is recorded in South Africa, what control measures would be appropriate? Which government agency should be responsible for devising and carrying out a control or containment programme? For example, the myrtle rust pathogen, P. psidii, was recorded for the first time in 2013 (Roux et al. 2013); this is a listed prohibited species in the NEM:BA A&IS regulations. The author is unaware of efforts led by any government agency to control this pathogen. Protocols or plans need to be developed and formulated by a government agency that has specifically been made responsible for control efforts. It may be noted here that despite a rapid response by Australian organs of state, the spread of this fungus was not halted in Australia (Carnegie et al. 2016; Pegg et al. 2014).
There is little information available on pathways of introduction, despite preventing introduction being in most cases the only practical means of control. It is recognised that most introductions have been human mediated (Fisher et al. 2012; Moralejo et al. 2009; Roy et al. 2014; Wingfield et al. 2001), but there is a need to identify potential routes and substrates of introductions. One of the most likely important means of introduction in the past was the transport of living crop plants growing in soil to our shores. The transport of live plants in the forestry and horticultural industries continues this trend (Moralejo et al. 2009; Roy et al. 2014). The nursery trade is also an important means of spreading alien fungi within countries. Bulk timber consignments, and timber products, are also important introduction pathways (Roy et al. 2014; Wingfield et al. 2001).
Currently, the importation of all organisms is controlled by DAFF, who administer applications for import by issuing permits, and through inspection and quarantine services. It is therefore important for cooperation between DEA and DAFF to ensure that risks to the environment are part of any risk assessment undertaken when considering an application for import. This should include applications for the import of microbial remedies such as Trichoderma species and B. bassiana, or mutualists such as AM fungi. In such cases, a precautionary principle may be appropriate, that developing commercial products using locally adapted indigenous strains of the species of interest would be preferable to importing alien strains. However, it needs to be recognised that in many cases we know so little about what fungi are native to South Africa, and their ecological importance, that a risk assessment may be impossible to compile. Standards for packing material and any medium in which plants, plant parts or animals are transported may also need modification.
There is a clear need to revise the current list of declared alien microbial species, as well as the list of prohibited species. It will be important at the same time to consider if any control options are possible against any listed species, as the NEM:BA act requires control to be implemented against these. The decision to include species should not only be based solely on the fact that they are recorded as alien but also on that a plan of action to at least ameliorate negative ecological impacts can be implemented.
Acknowledgements
The Department of Environmental Affairs: Natural Resources Management Programme (DEA:NRMP) is thanked for providing funding, and three unknown reviewers for their valuable comments which improved the text.
Competing interests
The author declares that he has no financial or personal relationship(s) that may have inappropriately influenced him in writing this article.
Authors' contributions
A.R.W. conceptualised this review, researched literature and wrote the manuscript.
References
Abad, Z.G., Abad, J.A., Cacciola, S.O., Pane, A., Faedda, R., Moralejo, E., et al., 2014, 'Phytophthora niederhauserii sp. nov., a polyphagous species associated with ornamentals, fruit trees and native plants in 13 countries', Mycologia 106, 431-447. https://doi.org/10.3852/12-119 [ Links ]
Adams, G.C., Rpux, J. & Wingfield, M.J., 2006, 'Cytospora species (Ascomycota, Diapothales, Valsaceae): introduced and native pathogens of trees in South Africa', Australasian Plant Pathology 35, 521-548. https://doi.org/10.1071/AP06058 [ Links ]
Adendorf, R., & Rijkenberg, F.H.J., 1995, 'New report on rust on Kikuyu grass in South Africa caused by Phakopsora apoda', Plant Disease 79, 1187. https://doi.org/10.1094/PD-79-1187B [ Links ]
Agrawal, A.A., Kotanenen, P.M., Mitchell, C.E., Power, A.G., Godsoe, W. & Klironomos, J., 2005, 'Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies', Ecology 86, 2979-2980. https://doi.org/10.1890/05-0219 [ Links ]
Allender, M.C., Raudabaugh, D.B., Gleason, F.H. & Miller, A.N., 2015, 'The natural history, ecology, and epidemiology of Ophidiomyces ophidiicola and its potential impact on free-ranging snake populations', Fungal Ecology 17, 187-196. https://doi.org/10.1016/j.funeco.2015.05.003 [ Links ]
Allsopp, N. & Holmes, P.M., 2001, 'The impact of alien plant invasion on mycorrhzas in mountain fynbos vegetation', South African Journal of Botany 67, 150-156. https://doi.org/10.1016/S0254-6299(15)31113-3 [ Links ]
Allsopp, N. & Stock, W.D., 1993, 'Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa', Bothalia 23, 91-104. [ Links ]
Anderson, P.K., Cunningham, A.A., Patel, N.G., Morales, F.J., Epstein, P.R. & Daszak, P., 2004, 'Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers', Trends in Ecology and Evolution 19, 535-544. https://doi.org/10.1016/j.tree.2004.07.021 [ Links ]
Bahramisharif, A., Lamprecht, S.C., Spies, C.F.J., Botha, W.J., Calitz, F.J. & McLeod, A., 2014, 'Pythium spp. associated with rooibos seedlings, and their pathogenicity towards rooibos, lupin and oat', Plant Disease 98, 223-232. https://doi.org/10.1094/PDIS-05-13-0467-RE [ Links ]
Balci, Y., Balci, S., Eggers, J., MacDonald, W.L., Juzwik, J., Long, R.P., et al., 2007, 'Phytophthora spp. associated with forest soils in eastern and north-central U.S. Oak ecosystems', Plant Disease 91, 705-710. https://doi.org/10.1094/PDIS-91-6-0705 [ Links ]
Bebber, D.P., Holmes, T. & Gurr, S.J., 2014, 'The global spread of crop pests and pathogens', Global Ecology and Biogeography 23, 1398-1407. https://doi.org/10.1111/geb.12214 [ Links ]
Becklin, K.M., Pallo, M. & Galen, C., 2012, 'Willows indirectly reduce arbuscular mycorrhizal fungal colonization in understorey communities', Journal of Ecology 100, 343-351. https://doi.org/10.1111/j.1365-2745.2011.01903.x [ Links ]
Beckstead, J., Meyer, S.E., Connolly, B.M., Huck, M.B. & Street, L.E., 2010, 'Cheatgrass facilitates spillover of a seed bank pathogen onto native grass species', Journal of Ecology 98, 168-177. https://doi.org/10.1111/j.1365-2745.2009.01599.x [ Links ]
Bennett, A.E. & Strauss, S.Y., 2013, 'Response to soil biota by native, introduced non-pest, and pest grass species: Is responsiveness a mechanism for invasion?' Biological Invasions 15, 1343-1353. https://doi.org/10.1007/s10530-012-0371-1 [ Links ]
Berger, L., Roberts, A.A., Voyles, J., Longcore, J.E., Murray, K.A. & Skerratt, L.F., 2016, 'History and recent progress on chytridiomycosis in amphibians', Fungal Ecology 19, 89-99. https://doi.org/10.1016/j.funeco.2015.09.007 [ Links ]
Bezuidenhout, C.M., Denman, S., Kirk, S.A., Botha, W.J., Mostert, L. & McLeod, A., 2010, 'Phytophthora taxa associated with cultivated Agathosma, with emphasis on the P. citricola complex and P. capensis sp. nov.', Persoonia 25, 32-49. https://doi.org/10.3767/003158510X538371 [ Links ]
Birnbaum, C. & Leishman, M.R., 2013, 'Plant-soil feedbacks do not explain invasion success of Acacia species in introduced range populations in Australia'. Biological Invasions 15, 2609-2625. https://doi.org/10.1007/s10530-013-0478-z [ Links ]
Blackwell, M., 2011, 'The fungi; 1, 2, 3, … 5.1 million species?' American Journal of Botany 98, 426-438. https://doi.org/10.3732/ajb.1000298 [ Links ]
Blaszkowski, J., Kovács, G.M., Balázs, T.K., Orlowska, E., Sadravi, M., Wubet, T., et al., 2010, 'Glomus africanum and G. iranicum, two new species of arbuscular mycorrhizal fungi (Glomeromycota)', Mycologia 102, 1450-1462. https://doi.org/10.3852/09-302 [ Links ]
Brasier, C.M., 2001, 'Rapid evolution of introduced plant pathogens via interspecific hybridization', BioScience 51, 123-133. https://doi.org/10.1641/0006-3568(2001)051[0123:REOIPP]2.0.CO;2 [ Links ]
Bottomley, A.M., 1948, 'Gasteromycetes of South Africa', Bothalia 4, 473-810. https://doi.org/10.4102/abc.v4i3.1859 [ Links ]
Cahill, D.M., Rookes, J.E., Wilson, B.A., Gibson, L. & McDougall, K.L., 2008, 'Phytophthora cinnamomi and Australia's biodiversity: Impacts, predictions and progress towards control', Australasian Journal of Botany 56, 279-310. https://doi.org/10.1071/BT07159 [ Links ]
Callaghan, S. & Guest, D., 2015, 'Globalisation, the founder effect, hybrid Phytophthora species and rapid evolution: New headaches for biosecurity', Australasian Plant Pathology 44, 255-262. https://doi.org/10.1007/s13313-015-0348-5 [ Links ]
Camilo-Alves, C.S.P., Clara, M.I.E. & Ribeiro, N.M.C.A., 2013, 'Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review', European Journal of Forest Research 132, 411-432. https://doi.org/10.1007/s10342-013-0688-z [ Links ]
Carnegie, A.J. & Cooper, K., 2011, 'Emergency response to the incursion of an exotic myrtaceous rust in Australia', Australasian Plant Pathology 40, 346-359. https://doi.org/10.1007/s13313-011-0066-6 [ Links ]
Carnegie, A.J., Kathuria, A., Peg, G.S., Entwistle, P., Nagel, M. & Giblin, F.R., 2016, 'Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia', Biological Invasions 18, 127-144. https://doi.org/10.1007/s10530-015-0996-y [ Links ]
Carvalho, L.M., Antunes, P.M., Martins-Loução, M.A. & Klironomous, J.N., 2010, 'Disturbance influences the outcome of plant-soil biota interactions in the invasive Acacia longifolia and in native species', Oikos 119, 1172-1180. https://doi.org/10.1111/j.1600-0706.2009.18148.x [ Links ]
Catford, J.A., Jansson, R. & Nilsson, C., 2009, 'Reducing reduncancy in invasion ecology by integrating hypotheses into a single theoretical framework', Diversity and Distributions 15, 22-40. https://doi.org/10.1111/j.1472-4642.2008.00521.x [ Links ]
Coetzee, M.P.A., Wingfield, B.D., Harrington, T.C., Steimel, J., Coutinho, T.A. & Wingfield, M.J., 2001, 'The root rot fungus Armillaria mellea introduced into South Africa by early Dutch settlers', Molecular Ecology 10, 387-396. https://doi.org/10.1046/j.1365-294x.2001.01187.x [ Links ]
Coetzee, M.P.A., Wingfield, B.D., Roux, J., Crous, P.W., Denman, S. & Wingfield, M.J., 2003, 'Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa', Plant Pathology 52, 604-612. https://doi.org/10.1046/j.1365-3059.2003.00879.x [ Links ]
Coetzee, J.C., 2010, 'Taxonomic notes on the Clathraceae (Phallales: Phallomycetidae) sensu Bottomley and a new key to the species in southern Africa', Bothalia 40, 155-159. https://doi.org/10.4102/abc.v40i2.205 [ Links ]
Collier, F.A. & Bidartondo, M.I., 2009, 'Waiting for fungi: The ectomycorrhizal invasion of lowland heathlands', Journal of Ecology 97, 950-963. https://doi.org/10.1111/j.1365-2745.2009.01544.x [ Links ]
Crous, P.W. & Braun, U., 1996, 'Cercosporoid fungi from South Africa', Mycotaxon 57, 233-321. [ Links ]
Crous, P.W., Groenewald, J.Z., den Breeÿen, A. & King, A., 2014, 'Cercosporella dolichandrae, Fungal Planet 243', Persoonia 32, 232-233. [ Links ]
Crous, P.W., Groenewald, J.Z. & Wood, A.R., 2013, 'Coniothyrium prosopidis & Peyronellaea prosopidis, Fungal Planet 165 & 166', Persoonia 31, 206-207. [ Links ]
Crous, P.W., Phillips, A.J.L. & Baxter, A.P., 2000, Phytopathogenic fungi from South Africa, University of Stellenbosch, Department of Plant Pathology Press, Stellenbosch. ISBN 0-7972-0777-5. [ Links ]
Crous, P.W., Rong, I.H., Wood, A.R., Lee, S., Glen, H., Botha, W., et al., 2006. 'How many species of fungi are there at the tip of Africa?' Studies in Mycology 55, 13-33. https://doi.org/10.3114/sim.55.1.13 [ Links ]
Cruywagen, E.M., Slippers, B., Roux, J. & Wingfield, M.J., 2016, 'Phylogenetic species recognition and hybridization in Lasiodiplodia: A case study on species from baobabs', Fungal Biology https://doi.org/10.1016/j.funbio.2016.07.014 [ Links ]
Cui, Q.-G. & He, W.-M., 2009, 'Soil Biota, but not soil nutrients, facilitate the invasion of Bidens pilosa relative to a native species Saussurea deltoidea', Weed Research 49, 201-206. https://doi.org/10.1111/j.1365-3180.2008.00679.x [ Links ]
Dawson, W., 2015, 'Release from belowground enemies and shifts in root traits as interrelated drivers of alien plant invasion success: A hypothesis', Ecology and Evolution 5, 4505-4516. https://doi.org/10.1002/ece3.1725 [ Links ]
Dawson, W. & Schrama, M., 2016, 'Identifying the role of soil microbes in plant invasions', Journal of Ecology 104, 1211-1218. https://doi.org/10.1111/1365-2745.12619 [ Links ]
Day, N.J., Dunfield, K.E. & Antunes, P.M., 2015, 'Temporal dynamics of plant-soil feedback and root associated fungal communities over 100 years of invasion by a non-native plant', Journal of Ecology 103, 1557-1569. https://doi.org/10.1111/1365-2745.12459 [ Links ]
Day, N.J., Dunfield, K.E. & Antunes, P.M., 2016, 'Fungi from a non-native invasive plant increase its growth but have different growth effects on native plants', Biological Invasions 18, 231-243. https://doi.org/10.1007/s10530-015-1004-2 [ Links ]
de la Peña, E., de Clerq, N., Bonte, D., Roiloa, S., Rodríguez-Echeverría, S. & Freitas, H., 2010, 'Plant-soil feedback as a mechanism of invasion by Carpobrotus edulis', Biological Invasions 12, 3637-3648. https://doi.org/10.1007/s10530-010-9756-1 [ Links ]
Desprez-Loustau, M.-L., Courtescuisse, R., Robin, C., Husson, C., Moreau, P.-A., Blancard, D., et al., 2010, 'Species diversity and drivers of spread of alien fungi (sensu lato) in Europe with particular focus on France', Biological Invasions 12, 157-172. https://doi.org/10.1007/s10530-009-9439-y [ Links ]
Desprez-Loustau, M.-L., Robin, C., Buée, M., Courtecuisse, R., Garbaye, J., Suffert, F., et al., 2007, 'The fungal dimension in biological invasions', TRENDS in Ecology and Evolution 22, 472-480. https://doi.org/10.1016/j.tree.2007.04.005 [ Links ]
Dickie, I.A., Bolstridge, N., Cooper, J.A. & Peltzer, D.A., 2010, 'Co-invasion by Pinus and its mycorrhizal fungi', New Phytologist 187, 475-484. [ Links ]
Dickie, I.A., Nuñez, M.A., Pringle, A., Lebel, T., Tourtellot, S.G. & Johnston, P.R., 2016, 'Towards management of invasive ectomycorrhizal fungi', Biological Invasions 18, 3383-3395. https://doi.org/10.1007/s10530-016-1243-x [ Links ]
Doidge, E.M., 1950, 'The South African fungi and lichens to the end of 1945', Bothalia 5, 1-1094. [ Links ]
Doidge, E.M. & Bottomley, A.M., 1931, 'A revised list of plant diseases occurring in South Africa', Botanical Survey of South Africa 11, 1-78. [ Links ]
Doidge, E.M., Bottomley, A.M., van der Plank, J.E. & Pauer, G.D., 1953, 'A revised list of plant diseases in South Africa', Science Bulletin 346, 1-122. [ Links ]
Duchicela, J., Vogelsang, K.M., Schultz, P.A., Kaonongbua, W., Middleton, E.L. & Bever, J.D., 2012, 'Non-native plants and soil microbes: Potential contributions to the consistent reduction in soil aggregate stability caused by the disturbance of North American grasslands', New Phytologist 196, 212-222. https://doi.org/10.111/j.1469-8137.2012.04233.x [ Links ]
Dunk, C.W., Lebel, T. & Keane, P.J., 2012, 'Characterization of ectomycorrhizal formation by the exotic fungus Amanita muscaria with Nothofagus cunninghamii in Victoria, Australia', Mycorrhiza 22, 135-147. https://doi.org/10.1007/s00572-011-0288-9 [ Links ]
Dunstan, W.A., Rudman, T., Shearer, B.L., Moore, N.A., Paap, T., Calver, M.C., et al., 2010, 'Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems', Biological Invasions 12, 913-925. https://doi.org/10.1007/s10530-009-9512-6 [ Links ]
Elgersma, K.J. & Ehrenfeld, J.G., 2011, 'Linear and non-linear impacts of a non-native plant invasion on soil microbial community structure and function', Biological Invasions 13, 757-768. https://doi.org/10.1007/s10530-010-9866-9 [ Links ]
Eppinga, M.B., Rietkerk, M., Dekker, S.C., De Ruiter, P.C. & Van der Putten, W.H., 2006, 'Accumulation of local pathogens: A new hypothesis to explain exotic plant invasions', Oikos 114, 168-176. https://doi.org/10.1111/j.2006.0030-1299.14625.x [ Links ]
Farr, D.F. & Rossman, A.Y., undated, Fungal databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA, viewed March - November 2016, from http://nt.ars-grin.gov/fungaldatabases/ [ Links ]
Feng, Y.-L., Li, Y.-P., Wang, R.-F., Callaway, R.M., Valiente-Banuet, A. & Inderjit, 2011, 'A quicker return energy-use strategy by populations of a subtropical invader in the non-native range: A potential mechanism for the evolution of increased competitive ability', Journal of Ecology 99, 1116-1123. https://doi.org/10.1111/j.1365-2745.2011.01843.x [ Links ]
Fisher, M.C., Henk, D.A., Briggs, C.J., Brownstein, J.S., Madoff, L.C., McCraw, S.L. et al., 2012, 'Emerging fungal threats to animal, plant and ecosystem health', Nature 484, 186-194. https://doi.org/10.1038/nature10947 [ Links ]
Flory, S.L. & Clay, K., 2013, 'Pathogen accumulation and long-term dynamics of plant invasions', Journal of Ecology 101, 607-613. https://doi.org/10.1111/1365-2745.12078 [ Links ]
Flory, S.L., Kleczewski, N. & Clay, K., 2011, 'Ecological consequences of pathogen accumulation on an invasive grass', Ecosphere 2, 120. https://doi.org/10.1890/ES11-00191.1 [ Links ]
Gaur, A., van Greuning, J.V., Sinclair, R.C. & Eicker, A., 1999, 'Arbuscular mycorrhizas of Vangueria infausta Burch. subsp. infausta (Rubiaceae) from South Africa', South African Journal of Botany 65, 434-436. https://doi.org/10.1016/S0254-6299(15)31036-X [ Links ]
Ganley, R.J. & Bulman, L.S., 2016, 'Dutch elm disease in New Zealand: Impacts from eradication and management programmes', Plant Pathology 65, 1047-1055. https://doi.org/10.1111/ppa.12527 [ Links ]
Gladieux, P., Feurtey, A., Hood, M.E., Snirc, A., Clavel, J., Dutech, C., et al., 2015, 'The population biology of fungal invasions', Molecular Ecology 24, 1969-1986. https://doi.org/10.1111/mec.13028 [ Links ]
Gorter, G.J.M.A., 1977, 'Index of plant pathogens and the diseases they cause in cultivated plants in South Africa', Science Bulletin 392, 1-177. [ Links ]
Gorter, G.J.M.A., 1993, 'A revised list of South African Erysiphaceae (powdery mildews) and their host plants', South African Journal of Botany 59, 566-568. https://doi.org/10.1016/S0254-6299(16)30671-8 [ Links ]
Greyling, I., Wingfield, M.J., Coetzee, M.P.A., Marincowitz, S. & Roux, J. 2016, 'The Eucalyptus shoot and leaf pathogen Teratosphaeria destructans recorded in South Africa', Southern Forests 2016, 1-7. https://doi.org/10.2989/20702620.2015.1136504 [ Links ]
Gryzenhout, M., 2010, Mushrooms of South Africa, Struik, Cape Town, South Africa. [ Links ]
Gundale, M.J., Kardol, P., Nilsson, M.-C., Nilsson, U., Lucas, R.W. & Wardle, D.A., 2014, 'Interactions with soil biota shift from negative to positive when a tree species is moved outside its native range', New Phytologist 202, 415-421. https://doi.org/10.1111/nph.12699 [ Links ]
Hagemann, G.D. & Rose, P.D., 1988, 'Leaf spot and blight on Acacia longifolia caused by Cylindrocladium scoparium: A new host record', Phytophylactica 20, 311-316. [ Links ]
Halbritter, A.H., Carroll, G.C., Güsewell, S. & Roy, B.A., 2012, 'Testing assumptions of the enemy release hypothesis: Generalist versus specialist enemies of the grass Brachypodium sylvaticum', Mycologia 104, 34-44. https://doi.org/10.3852/11-071 [ Links ]
Hale, A.N., Lapointe, L. & Kalisz, S., 2016, 'Invader disruption of belowground plant mutualisms reduces carbon acquisition and alters allocation patterns in a native forest herb', New Phytologist 209, 542-549. https://doi.org/10.1111/nph.13709 [ Links ]
Hansen, E.M., 2008, 'Alien forest pathogens: Phytophthora species are changing world forests', Boreal Environment Research 13, 33-41. [ Links ]
Hansen, E.M., Goheen, D.J., Jules, E.S. & Ullian, B., 2000, 'Managing Port-Orford-Cedar and the introduced pathogen Phytophthora lateralis', Plant Disease 84, 4-10. https://doi.org/10.1094/PDIS.2000.84.1.4 [ Links ]
Hardy, G.E.St.J., Barrett, S. & Shearer, B.L., 2001, 'The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems', Australasian Plant Pathology 30, 133-139. https://doi.org/10.1071/AP01012 [ Links ]
Hatting, J.L., Humber, R.A., Poprawski, T.J. & Miller, R.M., 1999, 'A survey of fungal pathogens of aphids from South Africa, with special reference to cereal aphids', Biological Control 16, 1-12. https://doi.org/10.1006/bcon.1999.0731 [ Links ]
Hatting, J.L., Poprawski, T.J. & Miller, R.M., 2000, 'Prevalences of fungal pathogens and other natural enemies of cereal aphids (Homoptera: Aphididae) in wheat under dryland and irrigated conditions in South Africa', BioControl 45, 179-199. https://doi.org/10.1023/A:1009981718582 [ Links ]
Hawksworth, D., 1991, 'The fungal dimension of biodiversity: Magnitude, significance, and conservation', Mycological Research 95, 641-655. https://doi.org/10.1016/S0953-7562(09)80810-1 [ Links ]
Hawksworth, D., 2001, 'The magnitude of fungal diversity: The 1.5 million species estimate revisited', Mycological Research 105, 1422-1432. https://doi.org/10.1017/S0953756201004725 [ Links ]
Hawley, G.L. & Dames, J.F., 2004, 'Mycorrhizal status of indigenous tree species in a forest biome of the Eastern Cape, South Africa', South African Journal of Science 100, 633-637. [ Links ]
Hawley, G.L., Taylor, A.F.S. & Dames, J.F., 2008, 'Ectomycorrhizas in association with Pinus patula in Sabie, South Africa', South African Journal of Science 104, 273-283. [ Links ]
Heath, R.N., Gryzenhout, M., Roux, J. & Wingfield, M.J., 2006, 'Discovery of the canker pathogen Chrysopothe austroafricana on native Syzygium spp. in South Africa', Plant Disease 90, 433-438. https://doi.org/10.1094/PD-90-0433 [ Links ]
Hill, S.B. & Kotanen, P.M., 2012, 'Biotic interactions experienced by a new invader: Effects of its close relatives at the community scale', Botany 90, 35-42. https://doi.org/10.1139/B11-084 [ Links ]
Hill, T.C.J., Tippert, J.T. & Shearer, B.L., 1995, 'Evaluation of three treatments for eradication of Phytophthora cinnamomi from deep, leached sands in southwest Australia', Plant Disease 79, 122-127. https://doi.org/10.1094/PD-79-0122 [ Links ]
Hoffman, M.T. & Mitchell, D.T., 1986, 'The root morphology of some legume spp. in the south-western Cape and the relationship of vesicular-arbuscular mycorrhizas with dry mass and phosphorus content of Acacia saligna seedlings', South African Journal of Botany 52, 316-320. https://doi.org/10.1016/S0254-6299(16)31527-7 [ Links ]
Huchzermeyer, K.D.A. & Van der Waal, B.C.W., 2012, 'Epizootic ulcerative syndrome: Exotic fish disease threatens Africa's aquatic ecosystems', Journal of the South African Veterinary Association 83, Art.#204, 1-6. https://doi.org/10.4102/jsava.v83i1.204 [ Links ]
Hynson, N.A., Merckx, V.S.F.T., Perry, B.A. & Treseder, K.K., 2013, 'Identities and distributions of the co-invading ectomycorrhizal fungal symbionts of exotic pines in the Hawaiian Islands', Biological Invasions 15, 2373-2385. https://doi.org/10.1007/s10530-013-1458-3 [ Links ]
Jacobsen, A.L., Roets, F., Jacobs, S.M., Esler, K.J. & Pratt, R.B., 2012, 'Dieback and mortality of South African fynbos shrubs is likely driven by a novel pathogen and pathogen-induced hydraulic failure', Austral Ecology 37, 227-235. https://doi.org/10.1111/j.1442-9993.2011.02268.x [ Links ]
Jairus, T., Mpumba, R., Chinoya, S. & Tedersoo, L., 2011, 'Invasion potential and host shifts of Australian and African ectomycorrhizal fungi in mixed eucalypt plantations', New Phytologist 192, 179-187. https://doi.org/10.1111/j.1469-8137.2011.03775.x [ Links ]
Kamgan, N.G., Jacobs, K., de Beer, Z.W., Wingfield, M.J. & Roux J., 2008, 'Ceratocystis and Ophiostoma species, including three new taxa, associated with wounds on native South African trees', Fungal Diversity 29, 37-59. [ Links ]
Knevel, I.C., Lans, T., Menting, F.B.J., Hertling, U.M. & van der Putten, W., 2004, 'Release from native root herbivores and biotic resistance by soil pathogens in a new habitat both affect the alien Ammophila arenaria in South Africa', Oecologia 141, 502-510. https://doi.org/10.1007/s00442-004-1662-8 [ Links ]
Koch, A.M., Antunes, P.M., Barto, E.K., Cipollini, D., Mummey, D.L. & Klironomos, J.N., 2011, 'The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity', Biological Invasions 13, 1627-1639. https://doi.org/10.1007/s10530-010-9920-7 [ Links ]
Kotzé, L.J.D., Wood, A.R. & Lennox, C.L., 2015, 'Risk assessment of the Acacia cyclops dieback pathogen, Pseudolagarobasidium acaciicola, as a mycoherbicide in the South African strandveld and limestone fynbos', Biological Control 82, 52-60. https://doi.org/10.1016/j.biocontrol.2014.12.011 [ Links ]
Kourtev, P.S., Ehrenfeld, J.G. & Häggblom, M., 2003, 'Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities', Soil Biology & Biochemistry 35, 895-905. https://doi.org/10.1016/S0038-071(03)00120-2 [ Links ]
Lankau, R., 2010, 'Soil microbial communities after allelopathic competition between Alliaria petiolate and a native species', Biological Invasions 12, 2059-2068. https://doi.org/10.1007/s10530-009-9608-z [ Links ]
Lankau, R.A., Bauer, J.T., Anderson, M.R. & Anderson, R.C., 2014, 'Long-term legacies and partial recovery of mycorrhizal communities after invasive plant removal', Biological Invasions 16, 1979-1990. https://doi.org/10.1007/s10530-014-0642-0 [ Links ]
Lee, D.H., Roux, J., Wingfield, B.D., Barnes, I. & Wingfield, M.J., 2016, 'New host range and distribution of Ceratocystis pirilliformis in South Africa', European Journal of Plant Pathology 146, 483-496. https://doi.org/10.1007/s10658-016-0933-7 [ Links ]
Li, H., Zhang, X., Zheng, R., Li, X., Elmer, W.H., Wolfe, L.M., et al., 2014, 'Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native slatmarsh plants in China', Journal of Ecology 102, 1112-1119. https://doi.org/10.1111/1365-2745.12285 [ Links ]
Linde, C., Drenth, A. & Wingfield, M.J., 1999, 'Gene and genotypic diversity of Phytophthora cinnamomi in South Africa and Australia revealed by DNA polymorphisms', European Journal of Plant Pathology 105, 667-680. https://doi.org/10.1023/A:1008755532135 [ Links ]
Litchman, E., 2010, 'Invisible invaders: Non-pathogenic invasive microbes in aquatic and terrestrial ecosystems', Ecology Letters 13, 1560-1572. https://doi.org/10.1111/j.1461-0248.2010.01544.x [ Links ]
Loo, J.A., 2009, 'Ecological impacts of non-indigenous invasive fungi as forest pathogens', Biological Invasions 11, 81-96. https://doi.org/10.1007/s10530-008-9321-3 [ Links ]
Lübbe, W.A. & Mostert, G.P., 1991, 'Rate of Ocotea bullata decline in association with Phytophthora cinnamomi at three study sites in the southern Cape indigenous forests', South African Forestry Journal 159, 17-24. https://doi.org/10.1080/00382167.1991.9630390 [ Links ]
MacKay, J. & Kotanen, P.M., 2008, 'Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area', Journal of Ecology 96, 1152-1161. https://doi.org/10.1111/j.1365-2745.2008.01426.x [ Links ]
Mangla, S., Inderjit & Callaway, R.M., 2008, 'Exotic invasive plant accumulates native soil pathogens which inhibit native plants', Journal of Ecology 96, 58-67. https://doi.org/10.111/j.1365-2745.2007.01312.x [ Links ]
Maoela, M.A., Jacobs, S.M., Roets, F. & Esler, K.J., 2016, 'Invasion, alien control and restoration: Legacy effects linked to folivorous insects and phylopathogenic fungi', Austral Ecology https://doi.org/10.1111/aec.12383 [ Links ]
Marincowitz, S., Groenewald, J.Z., Wingfield, M.J. & Crous, P.W., 2008, 'Species of Botryosphaeriaceae occurring on Proteaceae', Persoonia 21, 111-118. https://doi.org/10.3767/003158508X372387 [ Links ]
Martin, F., Díez, J., Dell, B. & Delaruelle, C., 2002, 'Phylogeography of the ectomycorrhizal Pisolithus species as inferred from nuclear ribosomal DNA ITS sequences', New Phytologist 153, 345-357. https://doi.org/10.1046/j.0028-646X.2001.00313.x [ Links ]
McLeod, A., Botha, W.J., Meitz, J.C., Spies, C.F.J., Tewoldemedhin, Y.T. & Mostert, L., 2009, 'Morphological and phylogenetic analysis of Pythium species in South Africa', Mycological Research 113, 933-951. https://doi.org/10.1016/j.mycres.2009.04.009 [ Links ]
McTaggart, A.R., Doungsa-ard, C. & Wingfield, M.J., 2015, 'Uromycladium acacia, the cause of a sudden, severe disease epidemic on Acacia mearnsii in South Africa', Australasian Plant Pathology 44, 637-645. https://doi.org/10.1007/s13313-015-0381-4 [ Links ]
Mehl, J.W.M., Slippers, B., Roux, J. & Wingfield, M.J., 2016, 'Overlap of latent pathogens in the Botryosphaeriaceae on a native and agricultural host', Fungal Biology https://doi.org/10.1016/j.funbio.2016.07.015 [ Links ]
Moralejo, E., Pérez-Sierra, A.M., Álvarez, L.A., Belbahri, L., Lefort, F. & Descals, E., 2009, 'Multiple alien Phytophthora taxa discovered on disease ornamental plants in Spain', Plant Pathology 58, 100-110. https://doi.org/10.1111/j.1365-3059.2008.01930.x [ Links ]
Morin, L., van der Merwe, M., Hartley, D. & Müller, P., 2009, 'Putative natural hybrid between Puccinia lagenophorae and an unknown rust fungus on Senecio madagascariensis in KwaZulu-Natal, South Africa', Mycological Research 113, 725-736. https://doi.org/10.1016/j.mycres.2009.02.008 [ Links ]
Morris, M.J., 1983, 'Evaluation of field trials with Colletotrichum gloeosporiodes for the biological control of Hakea sericea', Phytophylactica 15, 13-16. [ Links ]
Morris, M.J., 1989. 'A method for controlling Hakea sericea Schad. seedlings using the fungus Colletotrichum gloeosporiodes (Penz.) Sacc.', Weed Research 29, 449-454. https://doi.org/10.1111/j.1365-3180.1989.tb01317.x [ Links ]
Morris, M.J., 1990, 'Cercospora priaropi recorded on the aquatic weed, Eichhornia crassipes, in South Africa', Phytophylactica 22, 255-256. [ Links ]
Morris, M.J., 1991, 'The use of plant pathogens for biological weed control in South Africa', Agriculture, Ecosystems and Environment 37, 239-255. https://doi.org/10.1016/0167-8809(91)90153-O [ Links ]
Morris, M.J. & Crous, P.W., 1994, 'New and interesting records of South African fungi. XIV. Cercosporoid fungi from weeds', South African Journal of Botany 60, 325-332. https://doi.org/10.1016/S0254-6299(16)30587-7 [ Links ]
Morris, M.J., Wingfield, M.J. & De Beer, C., 1993, 'Gummosis and wilt of Acacia mearnsii in South Africa caused by Ceratocystis fimbriata', Plant Pathology 42, 814-817. https://doi.org/10.1111/j.1365-3059.1993.tb01570.x [ Links ]
Morris, M.J., Wood, A.R. & den Breeÿen, A., 1999, 'Plant pathogens and biological control of weeds in South Africa: A review of projects and progress during the last decade', African Entomology memoir 1, 129-137 [ Links ]
Nakabonge, G., Gryzenhout, M., Roux, J., Wingfield, B.D. & Wingfield, M.J., 2006, 'Celoporthe dispersa gen. et sp. nov. from native Myrtales in South Africa', Studies in Mycology 55, 255-267. https://doi.org/10.3114/sim.55.1.255 [ Links ]
Nesamari, R., Coutinho, T.A. & Roux, J., 2016, 'Investigations into Encephalartos insect pests and diseases in South Africa and identification of Phytophthora cinnamomi as a pathogen of the Mojadji cycad', Plant Pathology. https://doi.org/10.1111/ppa.12619 [ Links ]
Niu, H.B., Liu, W.X., Wan, F.H. & Liu, B., 2007, 'An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives', Plant Soil 294, 73-85. https://doi.org/.1007/s11104-007-9230-8 [ Links ]
Nkuekam, G.K., Barnes, I., Wingfield, M.J. & Roux, J., 2009, 'Distribution and population diversity of Ceratocystis pirilliformis in South Africa', Mycologia 101, 17-25. https://doi.org/10.3852/07/171 [ Links ]
Nuñez, M.A. & Dickie, I.A., 2014, 'Invasive belowground mutualists of woody plants', Biological Invasions 16, 645-661. https://doi.org/10.1007/s10530-013-0612-y [ Links ]
O'Donnell, K., Rooney, A.P., Mills, G.L., Kuo, M. & Weber, N.S., 2011, 'Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic', Fungal Genetics and Biology 48, 252-265. https://doi.org/10.1016/j.fgb.2010.09.006 [ Links ]
Owen, S.M., Sieg, C.H., Johnson, N.C. & Gehring, C.A., 2013, 'Exotic cheatgrass and loss of soil biota decrease the performance of a native grass', Biological Invasions 15, 2503-2517. https://doi.org/10.1007/s10530-013-0469-0 [ Links ]
Pattison, Z., Rumble, H., Tanner, R.A., Jin, I. & Gange, A.C., 2016, 'Positive plant-soil feedbacks of the invasive Impatiens glandulifera and their effects on above-ground microbial communities', Weed Research 56, 198-207. https://doi.org/10.1111/wre.12200 [ Links ]
Pavlic, D., Slippers, B., Coutinho, T.A. & Wingfield, M.J., 2007, 'Botrysphaeriaceae occurring on native Syzygium cordatum in South Africa and their potential threat to Eucalyptus', Plant Pathology 56, 624-636. https://doi.org/10.1111/j.1365-3059.2007.01608.x [ Links ]
Pearson, A.A., 1950, 'Cape agarics and boleti', Transactions of the British Mycological Society 33, 276-316. https://doi.org/10.1016/S0007-1536(50)80080-3 [ Links ]
Pegg, G.S., Giblin, F.R., McTaggart, A.R., Guymer, G.P., Taylor, H., Ireland, K.B., et al., 2014, 'Puccinia psidii in Queensland, Australia: Disease symptoms, distribution and impact', Plant Pathology 63, 1005-1021. https://doi.org/10.1111/ppa.12173 [ Links ]
Philibert, A., Desprez-Loustau, M.-L., Fabre, B., Frey, P., Halkett, F., Husson, C., et al., 2011, 'Predicting invasion success of forest pathogenic fungi from species traits', Journal of Applied Ecology 48, 1381-1390. https://doi.org/10.1111/j.1365-2664.2011.02039.x [ Links ]
Pretorius, Z.A., Bender, C.M. & Visser, B., 2015, 'The rusts of wild rye in South Africa', South African Journal of Botany 96, 94-98. https://doi.org/10.1016/j.sajb.2014.10.005 [ Links ]
Pretorius, Z.A., van Wyk, P.S. & Kriel, W.M., 2000, 'Occurrence of Puccinia xanthii on sunflower in South Africa', Plant Disease 84, 924. https://doi.org/10.1094/PDIS.2000.84.8.924A [ Links ]
Pretorius, Z.A., Visser, B. & du Preez, P.J., 2007, 'First report of Asian Soybean Rust caused by Phakopsora pachyrhizi on Kudzu in South Africa', Plant Disease 91, 1364. https://doi.org/10.1094/PDIS-91-10-1364C [ Links ]
Pringle, A., Adams, R.I., Cross, H.B. & Bruns, T.D., 2009, 'The ectomycorrhizal fungus Amanita phalloides was introduced and is expanding its range on the west coast of North America', Molecular Ecology 18, 817-833. https://doi.org/10.1111/j.1365-294X.2008.04030.x [ Links ]
Redecker, D. & Raab, P., 2006, 'Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): Recent developments and new gene markers', Mycologia 98, 885-895. https://doi.org/10.3852/mycologia.98.6.885 [ Links ]
Reinhart, K.O. & Callaway, R.M., 2006, 'Soil biota and invasive plants', New Phytologist 170, 445-457. https://doi.org/10.1111/j.1469-8137.2006.01715.x [ Links ]
Reinhart, K.O., Tytgat, T., Van der Putten, W.H. & Clay, K., 2010, 'Virulence of soil-borne pathogens and invasion by Prunus serotina', New Phytologist 186, 484-495. https://doi.org/10.1111/j.1469-8137.2009.03159.x [ Links ]
Richardson, D.M., Allsopp, N., D'Antonio, C.M., Milton, S.J. & Rejmánek, M., 2000, 'Plant invasions - The role of mutualisms', Biological Review 75, 65-93. https://doi.org/10.1017/S0006323199005435 [ Links ]
Rockett, T.R. & Kramer, C.L., 1974, 'Periodicity and total spore production by lignicolous basidiomycetes', Mycologia 66, 817-829. https://doi.org/10.2307/3758202 [ Links ]
Rodriguez-Echeverria, S., Teixeira, H., Correia, M., Timóteo, S., Heleno, R., Öpik, M. et al., 2016, 'Arbuscular mycorrhizal fungai communities from tropical Africa reveal strong ecological structure', New Phytologist 213, 380-390. https://doi.org/10.1111/nph.14122 [ Links ]
Rong, I.H. & Baxter, A.P., 2006, 'The South African National Collection of Fungi: Celebrating a centenary 1905-2005', Studies in Mycology 55, 1-12. https://doi.org/10.3114/sim.55.1.1 [ Links ]
Rong, I.H. & Grobbelaar, E., 1998, 'South African records of associations between fungi and arthropods', African Plant Protection 4, 43-63. [ Links ]
Roux, J. & Coetzee, M.P.A., 2005, 'First report of Pink Disease on native trees in South Africa and phylogenetic placement of Erythricium salmonicolor in the Homobasidiomycetes', Plant Disease 89, 1158-1163. https://doi.org/10.1094/PD-89-1158 [ Links ]
Roux, J., Germishuizen, I., Nadel, R., Lee, D.J., Wingfield, M.J. & Pegg, G.S., 2015, 'Risk assessment for Puccinia psidii becoming established in South Africa', Plant Pathology 64, 1326-1335. https://doi.org/10.1111/ppa.12380 [ Links ]
Roux, J., Granados, G.M., Shuey, L., Barnes, I., Wingfield, M.J. & McTaggart, A.R., 2016, 'A unique genotype of the rust pathogen, Puccinia psidii, on Myrtaceae in South Africa', Australasian Plant Pathology 45, 645-652. https://doi.org/10.1007/s13313-016-0447-y [ Links ]
Roux, J., Greyling, I., Coutinho, T.A., Verleur, M. & Wingfield, M.J., 2013, 'The myrtle rust pathogen, Puccinia psidii, discovered in Africa', IMA Fungus 4, 155-159. https://doi.org/10.5598/imafungus.2013.04.01.14 [ Links ]
Roux, J., Heath, R.N., Labuschagne, L., Nkuekam, G.K. & Wingfield, M.J., 2007, 'Occurrence of the wattle wilt pathogen, Ceratocystis albifundus on native South African trees', Forest Pathology 37, 292-302. https://doi.org/10.1111/j.1439-0329.2007.00507.x [ Links ]
Roy, B.A., Alexander, H.M., Davidson, J., Campbell, F.T., Burdon, J.J., Sniezko, R. et al., 2014, 'Increasing forest loss worldwide from invasive pests require new trade regulations', Frontiers in Ecology and Environment 12, 457-465. https://doi.org/10.1890/130240 [ Links ]
Scharfy, D., Güsewell, S., Gessner, M.O. & Venterink, H.O., 2010, 'Invasion of Solidago gigantean in contrasting experimental plant communities: Effects on soil microbes, nutrients and plant-soil feedbacks', Journal of Ecology 98, 1379-1388. https://doi.org/10.1111/j.1365-2745.2010.01722.x [ Links ]
Schoch, C.L., Crous, P.W., Wingfield, B.D. & Wingfield, M.J., 1999, 'The Cylindrocladium candelabrum species complex includes four distinct mating populations', Mycologia 91, 286-298. https://doi.org/10.2307/3761374. [ Links ]
Scholler, M., Lutz, M., Wood, A.R., Hagendorn, G. & Mennicken, M., 2011, 'Taxonomy and phylogeny of Puccinia lagenophorae: A study using rDNA sequence data, morphological and host range features', Mycological Progress 10, 175-187. https://doi.org/10.1007/s11557-010-0687-0 [ Links ]
Shearer, B.L., Byrne, A., Dillon, M. & Buehrig, R., 1997, 'Distribution of Armillaria luteobubalina and its impact on community diversity and structure in Eucalyptus wandoo woodland in southern Western Australia', Australian Journal of Botany 45, 151-165. https://doi.org/10.1071/BT95083 [ Links ]
Shearer, B.L. & Tippert, J.T., 1988, 'Distribution and impact of Armillaria luteobubalina in the Eucalyptus marginata forest in South-western Australia', Australian Journal of Botany 36, 433-445. https://doi.org/10.1071/BT9880433 [ Links ]
Shearer, B.L. & Tippert, J.T., 1989, Jarrah dieback: The dynamics and management of Phytophthora cinnamomi in the Jarrah (Eucalyptus marginata) forests of South-western Australia, Research Bulletin No. 3. Department of Conservation and Land Management, Como, Australia. ISSN 1032-8106. [ Links ]
Slippers, B. & Wingfield, M.J., 2007, 'Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact', Fungal Biology Reviews 21, 90-106. https://doi.org/10.1016/j.fbr.2007.06.002 [ Links ]
Sosnowski, M.R., Fletcher, J.D., Daly, A.M., Rodoni, B.C. & Viljanen-Rollinson, S.L.H., 2009, 'Techniques for the treatment, removal and disposal of host material during programmes for plant pathogen eradication', Plant Pathology 58, 621-635. https://doi.org/10.1111/j.1365-3059.2009.02042.x [ Links ]
Straker, C.J., Hilditch, A.J. & Rey, M.E.C., 2010, 'Arbuscular myccorhizal fungi associated with cassava (Manihot esculenta Crantz) in South Africa', South African Journal of Botany 76, 102-111. https://doi.org/10.1016/j.sajb.2009.09.005 [ Links ]
Straker, C.J., Weiersbye, I.M. & Witkowski, E.T.F., 2007, 'Arbuscular mycorrhiza status of gold and uranium tailings and surrounding soils of South Africa's deep level gold mines: I. Root colonization and spore levels', South African Journal of Botany 73, 218-225. https://doi.org/10.1016/j.sajb.2006.12.006 [ Links ]
Strauss, U., Human, H., Gauthier, L., Crewe, R.M., Dietemann, V. & Pirk, C.W.W., 2013, 'Seasonal prevalence of pathogens and parasites in the savannah honeybee (Apis mellifera scutellata)', Journal of Invertebrate Pathology 114, 45-52. https://doi.org/10.1016/j.jip.2013.05.003 [ Links ]
Stukenbrock, E.H., 2016, 'The role of hybridization in the evolution and emergence of new fungal plant pathogens', Phytopathology 106, 104-112. https://doi.org/10.1094/PHYTO-08-15-0184-RVW [ Links ]
Stutz, J.C., Copeman, R., Martin, C.A, & Morton, J.B., 2000, 'Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa', Canadian Journal of Botany 78, 237-245. https://doi.org/10.1139/b99-183 [ Links ]
Suding, K.N., Harpole, W.S., Fukami, T., Kulmatiski, A., MacDougall, A.S., Stein, C., et al. 2013, 'Consequences of plant-soil feedbacks in invasion', Journal of Ecology 101, 298-308. https://doi.org/10.1111/1365-2745.12057 [ Links ]
te Beest, M., Stevens, N., Olff, H. & van der Putten, W.H., 2009, 'Plant-soil feedback induces shift in biomass allocation in the invasive plant Chromolaena odorata', Journal of Ecology 97, 1281-1290. https://doi.org/10.1111/j.1365-2745.2009.01574.x [ Links ]
Tedersoo, L., Bahram, M., Põlme, S., Kõljalj, U., Yourou, N.S., Wijesundera, R., et al., 2014, 'Global diversity and geography of soil fungi', Science 346, 1256688. https://doi.org/10.1126/science.1256688 [ Links ]
Tomkins, D.M., Carver, S., Jones, M.E., Krkošek, M. & Skerratt, L.F., 2015, 'Emerging infectious diseases of wildlife: A critical perspective', Trends in Parasitology 31, 149-159. https://doi.org/10.1016/j.pt.2015.01.007 [ Links ]
Trappe, J.M., Claridge, A.W., Arora, D. & Smit, W.A., 2008, 'Desert truffles of the African Kalahari: Ecology, ethnomycology, and taxonomy', Economic Botany 62, 521-529. [ Links ]
Uhlmann, E., Görke, C., Petersen, A. & Oberwinkler. F., 2004, 'Arbuscular mycorrhizae from semiarid regions of Namibia', Canadian Journal of Botany 82, 645-653. https://doi.org/10.1139/B04-039 [ Links ]
Vacher, C., Daudin, J.-J., Piou, D. & Desprez-Loustau, M.-L., 2010, 'Ecological integration of alien species into a tree-parasitic fungus network', Biological Invasions 12, 3249-3259. https://doi.org/10.1007/s10530-010-9719-6 [ Links ]
van der Heijden, M.G.A., Martin, F.M., Selosse, M.-A. & Sanders, I.R., 2015, 'Mycorrhizal ecology and evolution: The past, the present, and the future', New Phytologist 205, 1406-1423. https://doi.org/10.1111/nph.13288 [ Links ]
van der Westhuizen, G.C.A. & Eicker, A., 1994, Field guide to Mushrooms of southern Africa, Struik, Cape Town, South Africa. [ Links ]
Van Grunsven, R.H.A., Bos, F., Ripley, B.S., Suehs, C.M. & Veenendaal, E.M., 2009, 'Release from soil pathogens plays an important role in the success of invasive Carpobrotus in the Mediterranean', South African Journal of Botany 75, 172-175. https://doi.org/10.1016/j.sajb.2008.09.003 [ Links ]
van Jaarsveld, L.C., Kriel, W.M. & Minaar, A., 2006, 'First report of Puccinia thaliae on Canna Lilly in South Africa', Plant Disease 90, 113. https://doi.org/10.1094/PD-90-0113C [ Links ]
van Kleunen, M. & Fischer, M., 2009, 'Release from foliar and floral fungal pathogen species does not explain the geographic spread of naturalized North American plants in Europe', Journal of Ecology 97, 385-392. https://doi.org/10.1111/j.1365-2745.2009.01483.x [ Links ]
van Wyk, P.S., 1973, 'Root and crown rot of silver trees', Journal of South African Botany 39, 255-260. [ Links ]
Vellinga, E.C., Wolfe, B.E. & Pringle, A., 2009, 'Global patterns of ectomycorrhizal introductions', New Phytologist 181, 960-973. https://doi.org/10.1111/j.1469-8137.2008.02728.x [ Links ]
Verbeeken, A. & Buyck, B., 2002, 'Diversity and ecology of tropical ectomycorrhizal fungi in Africa', in R. Watling, J.C. Frankland, A.M. Ainsworth, S. Isaac, & C.H. Robinson (eds.), Tropical mycology Vol. 1, Macromycetes, pp.11-24, CAB International, Wallingford, UK. [ Links ]
Vettraino, A.M., Morel, O., Perlerou, C., Robin, C., Diamandis, S. & Vannini, A., 2005, 'Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with ink disease and crown decline', European Journal of Plant Pathology 111, 169-180. https://doi.org/10.1007/s10658-004-1882-0 [ Links ]
Vitullo, D., De Curtis, F., Palmieri, D. & Lima, G., 2014, 'Milkwort (Polygala myrtifolia L.) decline is caused by Fusarium oxysporum and F. solani in Southern Italy', European Journal of Plant Pathology 140, 883-886. https://doi.org/10.1007/s10658-014-0514-6 [ Links ]
Vizzini, A., Zotti, M. & Mello, A., 2009, 'Alien fungal species distribution: The study case of Favolaschia calocera', Biological Invasions 11, 417-429. https://doi.org/10.1007/s10530-008-9259-5 [ Links ]
Von Broembsen, S.L., 1984a, 'Occurrence of Phytophthora cinnamomi on indigenous and exotic hosts in South Africa, with special reference to the south-western Cape Province', Phytophylactica 16, 221-225. [ Links ]
Von Broembsen, S.L., 1984b, 'Distribution of Phytophthora cinnamomi in rivers of the south-western Cape Province', Phytophylactica 16, 227-229. [ Links ]
Von Broembsen, S.L. & Brits, G.J., 1985, 'Phytophthora root rot of commercially cultivated Proteas in South Africa', Plant Disease 69, 211-213. https://doi.org/10.1094/PD-69-211 [ Links ]
Von Broembsen, S.L. & Kruger, F.J., 1985, 'Phytophthora cinnamomi associated with mortality of native vegetation in South Africa', Plant Disease 69, 715-717. https://doi.org/10.1094/PD-69-715 [ Links ]
Wehner, J., Powell, J.R., Muller, L.A.H., Caruso, T., Veresoglou, S.D., Hempel, S. et al., 2014, 'Determinants of root-associated fungal communities within Asteraceae in a semi-arid- grassland', Journal of Ecology 102, 425-436. https://doi.org/10.1111/1365-2745.12197 [ Links ]
Wingfield, M.J., Coetzee, M.P.A., Crous, P.W., Six, D. & Wingfield, B.D., 2010, 'Fungal phoenix rising from the ashes?' IMA Fungus 1, 149-153. https://doi.org/10.5598/imafungus.2010.01.02.06 [ Links ]
Wingfield, M.J., Slippers, B., Roux, J. & Wingfield, B.D., 2001, 'Worldwide movement of exotic forest fungi, especially in the tropics and the southern hemisphere', BioScience 51, 134-140. https://doi.org/10.1641/0006-3568(2001)051[0134:WMOEFF]2.0.CO;2 [ Links ]
Wood, A.R. & Ginns, J., 2006, 'A new dieback disease of Acacia cyclops in South Africa caused by Psuedolagarobasidium acaciicola sp. nov.', Canadian Journal of Botany 84, 750-758. https://doi.org/10.1039/B06-032 [ Links ]
Wood, A.R. & Scholler, M., 2002, 'Puccinia abrupta var. partheniicola on Parthenium hysterophorus in Southern Africa', Plant Disease 86, 327. https://doi.org/10.1094/PDIS.2002.86.3.327A [ Links ]
Xingjun, Y., Dan, Y., Zhijun, L. & Keping, M., 2005, 'A new mechanism of invader success: Exotic plant inhibits natural vegetation restoration by changing soil microbe community', Chinese Science Bulletin 50, 1105-1112. https://doi.org/10.1360/04WC0280 [ Links ]
Zachariades, C., Paterson, I.D., Strathie, L.W., Hill, M.P. & Wilgen, B.W.V., 2017, 'Assessing the status of biological control as a management tool for suppression of invasive alien plants in South Africa', Bothalia 47(2), a2142. https://doi.org/10.4102/abc.v47i2.2142 [ Links ]
Zhang, Q., Yang, R., Tang, J., Yang, H., Hu, S. & Chen, X., 2010, 'Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion', PLoS One 5, e12380. https://doi.org/10.1371/journal.pone.0012380 [ Links ]
 Correspondence:
Correspondence:
Alan R. Wood
wooda@arc.agric.za
Received: 05 July 2016
Accepted: 23 Dec. 2016
Published: 31 Mar. 2017
Note: This paper was initially delivered at the 43rd Annual Research Symposium on the Management of Biological Invasions in South Africa, Goudini Spa, Western Cape, South Africa on 18-20 May 2016.














