Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.47 n.2 Pretoria 2017
http://dx.doi.org/10.4102/abc.v47i2.2172
ORIGINAL RESEARCH
Changes in the composition and distribution of alien plants in South Africa: An update from the Southern African Plant Invaders Atlas
Lesley HendersonI; John R.U. WilsonII, III
IAgricultural Research Council - Plant Protection Research Institute, South Africa
IISouth African National Biodiversity Institute (SANBI), Kirstenbosch Research Centre, South Africa
IIICentre for Invasion Biology, Department of Botany and Zoology, Stellenbosch University, South Africa
ABSTRACT
BACKGROUND: Data on alien species status and occurrence are essential variables for the monitoring and reporting of biological invasions. The Southern African Plant Invaders Atlas (SAPIA) Project has, over the past 23 years, atlassed alien plants growing outside of cultivation.
OBJECTIVES: To document changes in the alien plant taxa recorded in SAPIA, assess trends in invasive distributions and explore effects of management and regulations.
METHOD: The numbers of alien plant taxa recorded were compared between May 2006 and May 2016, and changes in the extent of invasions at a quarter-degree squares (qds) scale were compared between 2000 and 2016. The effectiveness of regulations and interventions was assessed in terms of the relative change in the extent of invasions.
RESULTS: As of May 2016, SAPIA had records for 773 alien plant taxa, an increase of 172 since 2006. Between 2000 and 2016, the number of qds occupied by alien plants increased by ~50%, due both to ongoing sampling and to spread. Successful classical biological control programmes have reduced the rate of spread of some taxa and in a few cases have led to range contractions. However, other interventions had no detectable effect at a qds scale.
CONCLUSIONS: South Africa has a growing number of invasive alien plant species across an increasing area. More taxa should be listed under national regulations, but ultimately more needs to be done to ensure that management is strategic and effective. SAPIA is a valuable tool for monitoring alien plant status and should be developed further so that invasions can be accurately tracked over time.
Introduction
There has been an increasing emphasis on developing standard metrics to measure and report biodiversity change (Pereira et al. 2013). In particular, there has been a recent call for a standardised system to monitor biological invasions in a country using information on (1) alien species occurrence, (2) species alien status (status of a species as either alien or native) and (3) alien species impact (Latombe et al. in press), although metrics on invaded areas and dispersal pathways will also be required (McGeoch et al. 2016; Wilson et al. 2017a). In terms of countries reporting on alien species occurrence, Latombe et al. (in press) argued that there should be a modular approach such that as national observation and monitoring systems develop, they become increasingly sophisticated. Four key stages in the development of such a monitoring system were identified: from a national list of alien species, to the presence of alien species in priority sites, to estimates of the national extent and area occupied by species and finally to a network of long-term monitoring sites. South Africa is in the enviable situation of already having achieved the third of these stages through a long-running atlas project that has been recording information on the national extent of alien plants since 1994 - the Southern African Plant Invaders Atlas (SAPIA) (Henderson 1998a).
The Southern African Plant Invaders Atlas was launched in January 1994 to collate data on the distribution, abundance and habitat types of alien plants growing outside of cultivation in southern Africa (Henderson 1998a). The atlas region covers primarily South Africa, and to a much lesser extent, neighbouring countries. The SAPIA database incorporates records gathered by 670 participants since 1994, along with roadside surveys by the lead author (L.H.) since 1979 (Henderson 1989, 1991a, 1991b, 1992, 1998b, 2007; Henderson & Musil 1984; Wells, Duggan & Henderson 1980). The species lists and distribution data in the SAPIA database have provided baseline information for national projects on invasive alien plants, such as the Natural Resources Management Programmes (NRMP) of the Department of Environmental Affairs (DEA). It has also directly contributed to the listing of invasive plants under the Alien and Invasive Species Regulations of the National Environmental Management: Biodiversity Act, Act 10 of 2004 (NEM:BA A&IS Regulations) (Department of Environmental Affairs 2014a). The SAPIA database is a useful and functioning resource for the storage, management and verification of data and as such provides support to a number of applied initiatives, including biological control (Zachariades et al. 2017) and work on incursion response planning by the South African National Biodiversity Institute's Invasive Species Programme (SANBI's ISP; Wilson et al. 2013).
The first comprehensive overview of the SAPIA database was published in 2007 (Henderson 2007). This publication gave a listing of all taxa in the database up to May 2006 and comprehensive information on the geographical extent and abundance of all taxa from 1979 until the end of 2000. A total of 557 species or 601 taxa (species, infra-specific taxa and unidentified species) were listed, of which 97 were prominent invaders. The SAPIA database is not, however, the most comprehensive source of information on naturalised species in South Africa. The first compilation of naturalised plants was produced by Wells et al. (1986), and at least a further 500 naturalised species are known in South Africa from the literature and herbarium collections in South Africa (Germishuizen & Meyer 2003; POSA 2012).
The aims of this paper are to:
-
provide an updated list of alien plant taxa recorded in SAPIA and their invasion status in South Africa;
-
document changes in the recorded extent of alien plant taxa and assess factors that might be responsible for these changes;
-
provide support for decisions on national projects dealing with legislation and the control of invasive alien plants; and
-
provide recommendations for how SAPIA can be improved to support efforts to monitor and report on the status of biological invasions in the region.
Methods
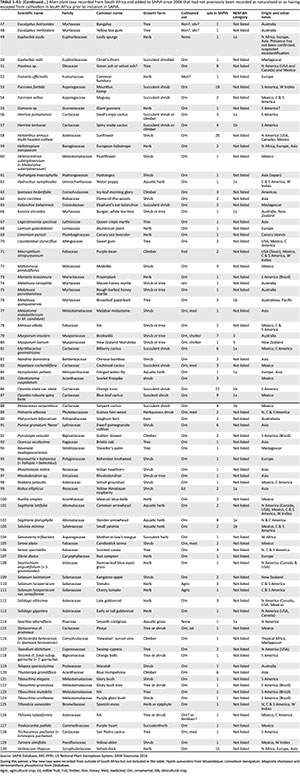
The SAPIA database is regularly updated with the latest copy of the database available from the lead author (L.H.) or from SANBI. This analysis was conducted using data collated in SAPIA up until the end of May 2016 (see Online Appendix 1 for the data here). Records were limited to alien plant taxa recorded as naturalised or as escapes from cultivation in South Africa, Lesotho and Swaziland. However, in a few instances, there are taxa that are not recorded from these three countries but are recorded as naturalised in neighbouring countries - these were noted. Current species and family names are mainly according to the Plant List (2013) and US National Plant Germplasm System: GRIN Taxonomy (2016).
Species introduction status
The previous review by Henderson (2007) provided data on taxa added to SAPIA up to May 2006; here, we examined the taxa that were added until May 2016 (i.e. over the course of a decade, Appendix 1). To look for taxonomic biases, we tested to see which families had significant changes in the number of taxa recorded in SAPIA relative to other families by calculating the probability using the hypergeometric distribution in R (R Core Team 2016). We corrected for multiple comparisons using the p.adjust function using the false discovery rate test (Benjamini & Hochberg 1995).
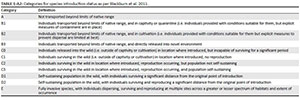
For a taxon to be recorded in SAPIA, it must have been growing outside of cultivation, but the population need not be invasive or have naturalised (sensu Blackburn et al. 2011, see also Appendix 2, Table 1-A2). To assess the link between SAPIA and the Blackburn Scheme, we compared information on the distribution and number of records in SAPIA with two recent detailed field evaluations: Jacobs et al. (2017) looked at Melaleuca spp. and N. Magona (unpublished data) did a similar exercise for Acacia spp.
Species distribution status
The Southern African Plant Invaders Atlas records come from several main sources - roadside surveys conducted by the lead author (L.H.), records from academics and managers specifically tasked with monitoring particular species (e.g. SANBI's ISP) and, finally, the general public using methods described in Henderson (2007). Roadside surveys by the lead author (L.H.) were conducted per 5-min square, using five qualitative abundance ratings [(1) rare: one sighting of one or a few plants; (2) occasional: a few sightings of one or a few plants; (3) frequent: many sightings of single plants or small groups; (4) abundant: many clumps or stands; and (5) very abundant: extensive stands]. Recently, all records are assigned a point locality with a note on precision and extent at the locale. As the aim of this paper was to look for broad-scale changes, and as the aim of SAPIA is to provide an atlas rather than detailed landscape level maps, we analysed distributions in terms of occupancy of quarter-degree squares (qds). To provide a comparison with the last review (Henderson 2007), distributions for the period up to 2000 were compared to those from the period from 2000 to May 2016. To limit bias, plant taxa that were added to the database based on records collected prior to 2000 but only collated after 2000 were not used in the analyses of changes in range.
To analyse changes in species distributions, we first looked at the changes between 2000 and 2016 with respect to how widespread plants were in 2000. There was no a priori reason to expect the relationship to be linear or log-linear, but initial assessments using general additive models indicated that the relationship was well described by a log-linear model. However, when these data were analysed using generalised linear models with Poisson errors, the residuals were heavily skewed. This was not surprising as there were numerous taxa that were in zero qds in 2000 but in several qds in 2016, while increases in the range of widespread taxa are limited by the size of the region (and more specifically, the number of qds that have suitable climate or habitat, e.g. Wilson et al. 2007). As such, we used a linear model with negative binomial errors (function glm.nb in the MASS library, Venables & Ripley 2002). Given the relationship is actually bounded, it might be expected to overestimate possible increases in range sizes, but checks of the fit of the model indicated that it was within acceptable ranges.
We then determined which taxa showed the greatest increase in their recorded ranges by examining the residuals from the fitted model. This provided an objective ranking of taxa in terms of increases in distributions relative to each other (Appendix 3, Table 1-A3). However, these increases are influenced by the focus of the sampling, which changed over the period under investigation. Prior to 2000, herbaceous taxa were largely excluded from SAPIA [except for about 33 species; most of which were listed as declared weeds under the Conservation of Agricultural Resources Act (CARA)]. Between 2000 and 2016, the curator of SAPIA (the lead author, L.H.) decided to more systematically record agricultural weeds and herbaceous taxa associated with human disturbance. Using expert opinion, taxa were classified as those that were known to have been under-recorded prior to 2000 and omitted from the analysis to determine a list of taxa that have spread most over the period (as opposed to those that have simply been sampled more).
To explore the impact of survey effort on distribution changes, we also compared changes in the distribution of species that have been the focus of intense survey effort over this period. The taxa selected were part of active projects to determine eradication feasibility by the SANBI's ISP (see Table 1 in Wilson et al. 2013).
Effectiveness of interventions and regulations
The NEM:BA A&IS Lists are based on the current impact and the future threat that species pose to South Africa (see Appendix 2, Table 2-A2 for a description of the different regulatory categories). Nonetheless, one would expect a link between the regulatory categories, status and extent (Parker et al. 1999 but see Hulme 2012). We first plotted range size against the regulatory status, and then tested to see if adding regulatory status as a factor to the model would have an impact on model fit.
Similarly, to explore the impact of management on the observed changes in distribution, we compared listed taxa that have been subject to clearing operations by the DEA's NRMP between 2000 and 2012 (A. Wannenburgh, unpublished information, notes on each taxa are in Online Appendix 1) to listed taxa that have not been subject to clearing.
The efficacy of biological control programmes has been assessed for all plants targeted using a standard system (Klein 2011; Moran, Hoffmann & Hill 2011a; Zachariades et al. 2017; see Appendix 2, Table 3-A2 for a list of the descriptions). To assess whether the success of biological control has also had an impact on alien plant distributions, we first looked to see if taxa that were under 'complete' biological control have shown less of an increase in range over the period 2000-2016 by adding this as a factor in the model. Second, to assess the impact in more depth, we looked at two plant groups that have been subjected to long-standing, highly successful and well-monitored biological control programmes - Australian acacias (Impson et al. 2011), and six invasive aquatic species that have been intensively surveyed by biological control researchers at the Agricultural Research Council (ARC) and Rhodes University over the past decade (Hill & Coetzee 2017).
Results
The data set extracted from SAPIA and used in this analysis is available as Online Appendix 1.
Species introduction status
There were 773 taxa (species, infra-specific taxa and combined taxa) catalogued in the SAPIA database from South Africa as of May 2016. This represents an increase in 172 taxa since May 2006 (although due to changes in nomenclature the true increase is slightly different, see discussion). Of these new taxa, 130 have no prior records in POSA (2012), Germishuizen and Meyer (2003) or Wells et al. (1986) (see Appendix 1 for a full list of new taxa); 73 of these have only been recorded in one qds, whereas 14 have been recorded in more than five qds. There were an additional nine taxa that have been recorded in SAPIA from neighbouring countries but not as yet in South Africa (see Online Appendix 1).
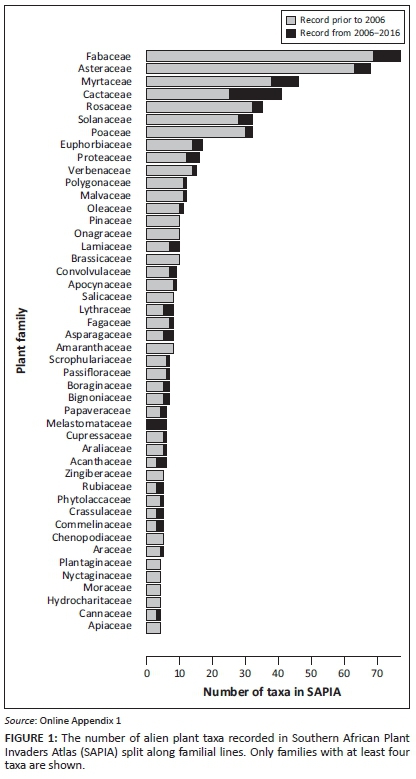
The families with the most new taxa recorded since 2006 were Cactaceae with 16 taxa, followed by Fabaceae and Myrtaceae with 8 taxa each; Melastomataceae and Asteraceae have 6 and 5 new taxa, respectively (Figure 1). Significantly more taxa in Berberidaceae, Cactaceae, Ericaceae, Melastomataceae and Polypodiaceae were added in this period to SAPIA when compared with other families, and relatively fewer taxa of Asteraceae and Fabaceae were added. Both Cactaceae and Melastomataceae had significantly more additions after correcting for multiple comparisons.
The introduction status of taxa as determined by dedicated and detailed field surveys shows that the extent as captured by SAPIA provides a fairly good indication of introduction status (Appendix 4). Taxa recorded in SAPIA from multiple sites are almost invariably category E under Blackburn et al.'s (2011) scheme. All taxa that were found to have naturalised populations were listed in SAPIA, and only a few taxa with naturalised populations had not yet been added to SAPIA (although they would be based on these field observations). However, for taxa recorded from only a few sites, detailed field evaluations will be required to confirm the extent of naturalisation and invasion.
Species distribution status
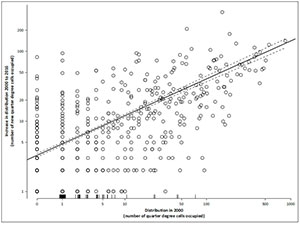
As of May 2016, SAPIA contained 87 000 records. As there were often multiple records of a species from any qds, the number of instances of an alien plant being present in a qds was 26 554. Between 2000 and 2016, there were 9069 instances where a taxon was found in a new qds, although if under-recorded taxa are excluded, there are 7221 instances [207 taxa are under-recorded in the SAPIA database and may be better documented in POSA and Germishuizen and Meyer (2003), see Online Appendix 1 for details]. This represents approximately a 43% increase in range since 2000 (~50% if previously under-recorded taxa are included). This does not, however, reflect instances where taxa have disappeared from a qds over this period (e.g. aquatic weeds), and these figures do not include taxa that were added to SAPIA based on records prior to 2000 (as the figure at that time was not known).
A generalised linear model with negative binomial errors provided a good fit to the data on the increase in distribution over the period 2000-2016 as a function of distribution in 2000 (Figure 2, Appendix 5). Taxa with small ranges in 2000 have seen their broad-scale distributions increase on average by up to fivefold over the intervening 16 years, while very widespread taxa have on average increased by 10%. By comparison, the annual rate of spread of alien trees at a landscape scale in South Africa is often estimated in the region of 4% - 8% when projecting costs (e.g. van Wilgen et al. 2016). It is not clear whether these differences are due to different rates of spread at different scales, or due to SAPIA still having under-sampled widespread taxa; but clearly, there is a very large amount of variation and using a single figure for spread in any model is highly questionable.
The list of taxa that have shown the greatest relative increase in range size is shown in Table 1.
The 38 taxa targeted by SANBI ISP (i.e. taxa that have been the focus of intense survey effort) showed a significantly greater increase in range than other taxa (LR = 11.8, d.f. = 1, p < 0.01), although the interaction effect between range size in 2000 and SANBI ISP was not significant. This result was consistent even if under-recorded taxa were excluded (LR = 30.1, d.f. = 1, p < 0.01; notably four SANBI ISP targets are known to be under-recorded in SAPIA prior to 2000: Furcraea foetida, Harrisia balansae, Hydrilla verticillata and Paspalum quadrifarium). While the SANBI ISP targets are clearly not a random selection of taxa, this result supports the contention that while SAPIA provides a useful baseline, more intensive surveys are required to get accurate estimates of range sizes.
Effectiveness of interventions and regulations
A total of 379 terrestrial plant taxa or 378 species are listed under the NEM:BA A&IS Regulations. All listed taxa which are documented in SAPIA are noted in Online Appendix 1 (338 taxa in total). Of the 40 listed taxa which are not recorded in SAPIA, 26 are potentially invasive and have been listed as a precautionary measure, 11 are listed only for the sub-Antarctic's Prince Edward and Marion islands, and of the remaining 3, Ammophila arenaria is not recorded on SAPIA as SAPIA has not surveyed fore-dunes nor have any public reported the species, Nephrolepis exaltata is listed in the regulations due to a misidentification (it is recorded under the correct name, Nephrolepis cordifolia, in SAPIA) and Orobanche ramosa is a parasitic plant long known in the Western Cape but not recorded in SAPIA (Table 2). Around 44% of taxa recorded in SAPIA are listed, with newer additions to SAPIA less likely to have been listed (49% of taxa recorded in SAPIA prior to 2006 are regulated, whereas 18% of taxa recorded after 2006 are regulated).
There is a clear relationship between the listed category and how widespread alien plants are (LR = 178, d.f. = 4, p < 0.001; Figure 3). Category 1a taxa are similar in extent to non-listed taxa, category 1b taxa are much more widespread and curiously category 2 taxa (that can be grown under a permit) are the most widespread taxa. This might reflect the fact that taxa that are both useful and invasive have already been widely distributed for utilisation.
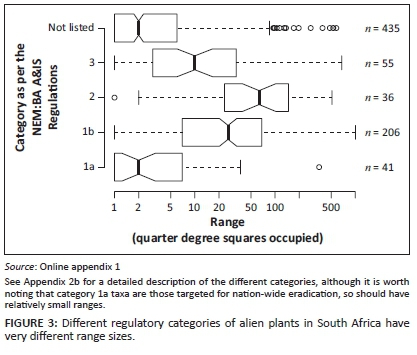
In terms of the link between regulatory status and the change in extent over the period 2000-2016, the interaction effect was not significant, although only just so (LR = 9.39, d.f. = 4, p = 0.0521). This is unsurprising, given the large differences in range size for the different regulatory categories (Figure 3). There was, however, a very large effect of regulatory status on observed rates of spread for those taxa which were not under-represented in SAPIA prior to 2000 (Appendix 5c). There were no significant differences between different categories of regulated taxa, but taxa in all regulated categories have spread much farther than non-listed taxa.
Approximately 126 taxa have been targeted for clearing by NRMP between 2000 and 2012. Most effort has been directed towards eight taxa, which make up 80% of the total condensed area treated. These taxa are: Solanum mauritianum (20%), Acacia mearnsii (14%), Prosopis spp. (14%), Acacia dealbata (9%), Pinus spp. (8%), Cereus jamacaru (7%), Lantana camara (4%) and Eucalyptus spp. (4%). Forty taxa make up 98% of the total condensed area treated. The remaining taxa make up 2% of the condensed area treated (Online Appendix 1). Targeting by DEA NRMP was not found to have a significant effect on the increase in broad-scale range of taxa relative to other taxa in SAPIA [e.g. when comparing a model with an interaction term between range size in 2000 and status as a major NRMP target with the base model with under-recorded taxa removed (i.e. Appendix 5b): LR = 3.69, d.f. = 2, p = 0.16].
Biological control programmes have been launched or are under investigation for 77 species, of which 13 species are rated as under complete control, 20 species under substantial control, 14 under negligible control, 1 under negligible to substantial control, 11 not determined and 18 under investigation (H. Klein, ARC-PPRI, pers. comm., July 2016) (Online Appendix 1). When the success of biological control was added to the model, the results were very complicated and not as expected. Taxa under complete control seemed to have actually spread farther relative to other taxa, whereas taxa under substantial (i.e. less control than complete) had spread less relative to other taxa. But a detailed interpretation was difficult as there was a significant interaction between range size in 2000, the level of success of the biological control and the spread observed. On closer inspection, it became clear that this was partly due to the fact that for some taxa that were under complete biological control, the agents responsible had only been released post-2000, that is, a plant taxon could have both spread rapidly and be contained by biological control in the period (Cylindropuntia fulgida var. mamillata, in particular). Therefore, we reran the analysis and compared taxa that were under complete or substantial biological control based on agents released prior to 2000 that caused considerable damage (sensu Klein 2011) against other taxa where there was a biological control programme in place, but if there was any success, it was later on (and so less likely to impact the pattern seen here). The result was much clearer. Alien plant taxa under successful biological control have spread much less than other alien plant taxa where biological control has been attempted (LR = 8.50, d.f. = 1, p = 0.0035). To put this in perspective, if a taxon was present in 100 qds in 2000, it would be expected to be in 127 qds by 2016 if biological control was successful, but 161 qds if biological control was not successful, that is, rates of spread were roughly halved.
Similarly, in terms of the specific biological control case-studies, Australian acacias that are under complete biological control appear to have shown much smaller increases in range than those that are not under complete control (Table 3). For the biological control of aquatic weeds, the results are even more impressive (Table 4). While there have been increases in the total number of qds that have ever been invaded, it is clear that the current range of several taxa has actually decreased over time.
Discussion
South Africa has a major alien plant invasion debt (Rouget et al. 2016). Well over a 100 new taxa have been recorded as naturalised or escapes from cultivation in the past decade and the recorded range of almost all plants has increased significantly. These observations are both cause for concern. However, it is also clear that there is a strong correlation between survey effort and both the number of naturalised plants detected and the extent of known invaders. Only for a very few taxa where explicit resources have been dedicated to their survey (e.g. Hill & Coetzee 2017; Wilson et al. 2013), can we be confident that their range is reasonably delimited at a qds scale. Thankfully, effective biological control (Zachariades et al. 2017) appears to have reduced rates of spread and have actually resulted in a contraction of the range of some taxa. Below, we discuss these and a few other results in more depth, make recommendations for improvements to the NEM:BA A&IS Regulations and conclude with how SAPIA can be improved in future.
Major increasing environmental threats
Species capable of invading and persisting in natural vegetation, and referred to as environmental weeds, pose the greatest threat to biodiversity. While an aim of this paper was to determine which taxa have spread the most since 2000 (i.e. Table 1), this does not give the complete picture of which taxa pose the greatest threats. The analyses presented here represent a starting point, but to fully assess and prioritise control programmes, information on potential future spread, field observations as to the impacts caused and the experiences of managers on the ground must be taken into account. We draw attention to nine taxa that we believe are of particular concern (Figure 4): Campuloclinium macrocephalum, Parthenium hysterophorus, Opuntia engelmannii, Cryptostegia grandiflora, Pennisetum setaceum, Tecoma stans, Sagittaria platyphylla, Gleditsia triacanthos and Trichocereus spachianus (see Zachariades et al. 2017 and references therein for a discussion on biological control of some of these taxa).
The species of most concern are those where biological control is not available, and that are not being contained by traditional control methods, in particular C. grandiflora, P. setaceum and T. spachianus. Gleditsia triacanthos, although it did not feature amongst the top 30 species in Table 1 because it has not been recorded at a high local abundance, almost doubled its extent from 111 to 216 qds and has the potential to become as troublesome as Prosopis spp. (Zachariades, Hoffmann & Roberts 2011) Biological control has only been partially effective against the Eastern Cape form of O. engelmannii (H. Klein, ARC-PPRI, pers. comm., July 2016). Biological control programmes are still at an early stage against C. macrocephalum, T. stans, P. hysterophorus and S. platyphylla. National species management programmes have been developed for some of these species (e.g. see Terblanche et al. 2016 for P. hysterophorus), but they are still to be implemented.
By contrast, the taxon that showed the greatest spread, C. fulgida var. mamillata, is not of particular concern as biological control has been extremely effective (Klein 2012) and has led to population collapse and death at all sites where the biological control agent, a cochineal, has been established (Xivuri et al. unpublished data). Similarly, there is effective biological control against Opuntia humifusa, although more work needs to be done to implement it.
It is perhaps not surprising that there was no evidence of DEA NRMP activities having reduced the rates of spread of targeted taxa. DEA NRMP control programmes are not strategic (van Wilgen et al. 2012), and as far as we are aware there have been no dedicated strategic efforts to contain specific invasive plants, or to reduce the rate at which they invade particular areas (see Le Maitre, Forsyth & Wilson 2015 for an example of a proposed strategy). By contrast, there is a clear signal that biological control has reduced the rates of spread.
Biological control as a method of limiting and reducing alien plant extents
Some species that have been the subjects of successful biological control programmes have shown very little expansion in their distribution areas in terms of qds occupied, and in general, successful biological control seems to be associated with a reduction in the rate of spread. In particular, the SAPIA distribution data presented here support the theory that seed-reducing agents are capable of slowing rates of spread and curbing expansion of invasive alien plant populations (Table 3). While it could be argued that these species have shown little expansion because they have almost reached the limits of their suitable range, models of their potential range indicate that there are still large suitable areas of the country as yet uninvaded (Rouget et al. 2004; Wilson et al. 2007), and the model used here takes starting range size into account and looks at changes relative to other taxa.
SAPIA also has good evidence of range contraction of Azolla filiculoides following the implementation of a biological control programme (Henderson 2011). Evidence of range contraction was made possible by intensive surveys prior to and following biological control by the researchers involved in the biological control programme (Coetzee et al. 2011, McConnachie, Hill & Byrne 2004). Up to the year 2000, shortly after the commencement of biological control, A. filiculoides had been recorded in 191 qds (see Table 4). By 2004, biological control led to the extirpation of A. filiculoides from the majority of sites surveyed (Coetzee et al. 2011). From 2001 to May 2016, it was recorded in only 24 new qds. Although the cumulative total qds is 215, it was actually recorded in 65 qds since 2001 (equivalent to a 66% contraction), and in only 14 qds since 2010 (equivalent to a 92% contraction). Aquatic weeds also showing contraction are Myriophyllum aquaticum with a 40% reduction and Eichhornia crassipes with a 15% contraction. Pistia stratiotes showed a slight expansion with almost as many qds up to 2000 as after 2000. Salvinia molesta showed the most expansion of 46%. Coetzee et al. (2011) still regard the programmes against P. stratiotes and S. molesta as successful but require better implementation of the programmes, with augmentative releases and re-distribution of agents. Azolla cristata, which has not been part of a formal biological control programme, showed the most expansion of 53%. This data set highlights the value of repeated monitoring at the same sites over time to determine trends.
New threats
The large number of cacti amongst the newly recorded taxa is probably partly the result of increased detection and awareness created by the national cactus working group (Kaplan et al., 2017), and it is apparent from field surveys that some taxa have probably been naturalised for decades, for example, C. fulgida var. mamillata (H.G. Zimmermann, Helmuth Zimmermann & Associates, pers. comm., July 2016). However, this also represents a new wave of cactus invasions arising from horticulture rather than agriculture (Novoa et al. 2015). Many new taxa in the Proteaceae and Myrtaceae have been recorded for the Western Cape and include species of Banksia, Callistemon and Melaleuca used in horticulture and floriculture. By contrast, there have been few recent introductions for forestry, so it is unsurprising that there were no new records of naturalisation in Pinaceae.
We expect that different threats will emerge as the dominant pathways of dispersal into and around the country change. In particular, there is the potential for spread between neighbouring countries (Faulkner et al. 2017). Some of the taxa that are invasive in neighbouring countries might already be in cultivation or might have had opportunities to spread but the climate is not suitable; but in some cases, spread of alien plants from neighbouring countries has been indicated as the primary source of invasions in South Africa (e.g. P. hysterophorus). A few species in SAPIA only recorded for Zimbabwe and Mozambique which are of concern are Hyptis suaveolens, Limnobium laevigatum and Vernonanthura phosphorica (Online Appendix 1). This is an issue that will clearly require greater international cooperation in biosecurity (Faulkner et al. 2017).
While it is concerning that taxa which have been assessed for their eradication feasibility appear to have spread significantly faster than other taxa, much of this is likely down to survey effort. All the SANBI ISP targets are the subject of active and passive surveillance programmes, often with the production of detailed risk maps and engagement with local stakeholders (e.g. Kaplan et al. 2014). As such, the current known distributions of SANBI ISP targets are expected to be much closer to the actual distributions - such a broad-scale delimitation is a pre-requisite for a successful incursion response (Wilson et al. 2017b). While this serves to again highlight the fact that many taxa in SAPIA are likely to be under-sampled, it is, however, also likely that many SANBI ISP targets are indeed spreading. The distribution of C. macrocephalum (pompom weed), for example, has increased rapidly over the past decade, and its spread has probably been exacerbated by various human-mediated dispersal vectors (McConnachie et al. 2011). Therefore, the 130 newly recorded taxa (Appendix 1) should be urgently screened for taxa where nation-wide eradication might be a feasible and desirable goal (category 1a under the NEM:BA A&IS Regulations). Of course, this is not to say that other taxa should not be likewise assessed. For example, Bartlettina sordida, which is not recorded in SAPIA, but is listed as category 1b, should be assessed for eradication feasibility and listing as category 1a. It is known to be in cultivation, but so far, there are no records of invasion. Evidence of its invasiveness in New Zealand (Breitwieser et al. 2010-2016), and its close relationship to other notoriously invasive species of the tribe Eupatoriae in the Asteraceae, such as Chromolaena odorata, C. macrocephalum and Ageratina adenophora, should make it a priority species for eradication.
Recommendations for changes to the NEM:BA A&IS Regulations
In this section, we provide some recommendations for changes to the NEM:BA A&IS Regulations, specifically: (1) several taxa should be listed or delisted, (2) there needs to be a formal process for dealing with the listing of taxa below the species level (e.g. subspecies and cultivars) and (3) there should be a separation between environmental and agricultural weeds.
SAPIA was used extensively to underpin the listing of taxa under the NEM:BA A&IS Regulations (Department of Environmental Affairs 2014a, 2014b) and should be used to inform updates of the lists. The NEM:BA A&IS Regulations were the result of an extensive process over a decade. However, there are some errors (cf. Wood 2017 for a discussion on the microbial lists), and the regulations recognise that the lists will need to be dynamic as the situation changes (there has already been one update as of February 2017). Species which could be considered for listing as 1b under NEM:BA because they are environmental weeds with the potential for much more spread include: Berberis aristata, Clusia rosea, Handroanthus chrysotrichus, Hypericum pseudohenryi, Manihot grahamii, Salvia coccinea, Thunbergia grandiflora, Tithonia tubaeformis, Verbascum thapsus and Verbena incompta. Some taxa which should be considered for listing under NEM:BA, but there are potential conflicts of interest with the horticultural and other industries, include Anigozanthos flavidus, Canna generalis, Gaura lindheimeri, Oenothera spp., Solidago spp. and Syzygium paniculatum. Species which are listed as 1a, but are already widespread in the country should be reclassified as 1b [e.g. Coreopsis lanceolata, F. foetida, Opuntia robusta (excluding spineless cultivars) and Tephrocactus articulatus]. There are also, however, some taxa which are listed for which there is no solid evidence that they are in the country (e.g. SANBI ISP has been trying for several field seasons to find Euphorbia esula without success, and it appears that the initial report might have been a mistake). Taxa which are listed under the regulations as 1a, 1b, 2 or 3 must have a physical herbarium record to prove that they are (or at least have been) in the country, and equally for taxa to be listed as prohibited, there should be a process for determining that the taxa really are not already present.
A major issue with the regulations is the need to deal with sub-specific entities, in particular, the horticultural industry is keen to ensure that cultivars of invasive taxa that pose an acceptable invasion risk should be exempt. However, even sterile cultivars can be invasive, for example, Opuntia aurantiaca can spread by detached stem sections and sterile fruits, and Vinca major can spread by rhizomes and stolons. The exemption of sterile cultivars of V. major nullifies its listing and allows nurseries to sell invasive plants. Currently, 'sterile' cultivars of 34 species are exempt (see Table 5), but there is no formal process for proving sterility. There is a common perception that plant sterility only means the inability to form viable seeds, but sexual reproduction in plants is dependent upon three major factors: the formation of fertile pollen, fertile embryo sacs and viable seeds (Spies & Du Plessis 1987). There needs to be a set protocol to determine the necessary and sufficient conditions for a plant taxon to be deemed an acceptable invasion risk on the basis of sterility, given that entities of the same species are proscribed. This should be based on a few principles: sterility must be such that the risk of invasions and impacts is acceptable; given closely related taxa have been shown to be invasive, the balance of evidence is no longer the same as for regulating a species, that is, the precautionary principle should apply; there needs to be a way in which sterile individuals can be differentiated from non-sterile individuals, that is, there needs to be a mechanism for implementation; and the process needs to be transparent, consistent and agreed by all stakeholders (cf. Zengeya et al. 2017). As such, it poses a scientific and regulatory challenge that will require some investment to address fully.
NEM:BA superseded CARA, which for many years was the only legislation dealing with weeds and invasive plants in South Africa. Currently, NEM:BA includes most of the taxa which were listed under CARA and this includes species which are mainly weeds of disturbed sites and agricultural lands, for example, species of Argemone, Cirsium, Datura and Xanthium. NEM:BA's prime concern is with environmental weeds and should exclude species which show limited ability to invade and persist in natural areas or undisturbed sites. Again, there should be a clear process for defining this cut-off as it might not be clear for many species. For example, Geerts et al. (2013) argued that Genista monspessulana currently poses a greater risk than Spartium junceum to the fynbos due to its greater ability to invade natural ecosystems, but the authors were not able to provide a mechanistic explanation for the differences between these two broom species. There should ideally be separate processes for listing taxa as environmental or agricultural threats although clearly some taxa will be both.
Limitations of the Southern African Plant Invaders Atlas database and recommendations for improvement
The SAPIA database has its limitations and users of the data need to be aware of these. Ideally, atlas data should be collected from the full extent of the atlas region, with a good measure of sampling intensity, within a specific time frame. The SAPIA database incorporates data that have been collected with varying sampling effort in space and time and could possibly qualify as an ad hoc dataset defined by Robertson, Cumming and Erasmus (2010).
The roadside surveys conducted by the author (L.H.) form the backbone of the SAPIA database, contributing about 60 000 of the total 87 000 records. These surveys followed a standard procedure but were rapid and lacked the sampling intensity of site inspection and are biased towards the more conspicuous trees and shrubs. Data received from SANBI's ISP since 2010 are mainly confined to potential and current eradication targets (Wilson et al. 2013). Records from the public are mostly ad hoc at specific sites. As a consequence of all these factors, the SAPIA database cannot provide accurate, up-to-date distribution data for all taxa across South Africa. For example, some species have shown little expansion, but they could have been underestimated, for example, Cestrum laevigatum with only 13% increase is easily overlooked as it blends into the natural vegetation. Nassella species are not easily detected during roadside surveys and no records were received from the public. Better data could be obtained if there were more data collectors spread across the atlas region, with a standardised recording procedure and sampling effort.
Recording absences (both from sites where the taxon has never been recorded and particularly from sites where it is no longer present) are essential if data are to be used for long-term monitoring and to track changes. In SAPIA, absence records have only been recorded for five aquatic species (see Table 4) as part of the Rhodes Invasive Aquatic Plants Surveys (Hill & Coetzee 2017). Dubious records, however, are queried. If an observer cannot provide satisfactory evidence (e.g. a photograph) to confirm a taxon's identity, then the record is not entered into SAPIA. Where identification is highly problematic, it might be necessary to submit a herbarium specimen for correct identification, although it is not practical or desirable for all SAPIA records to be linked to herbarium specimens. Other approaches to identification, for example, through the wisdom of the crowd (Silvertown et al. 2015), might be needed. But to achieve the recommendations of Latombe et al. (in press) for monitoring and reporting on biological invasions, there should be a process in addition to SAPIA whereby several long-term monitoring sites are established at which invasion dynamics of all taxa are documented in detail.
Similarly, while SAPIA provides some indication of the introduction status of species, a separate process is required to list all alien plants in the country and to confirm their status along the introduction-naturalisation-invasion continuum as per Blackburn et al. (2011). There have been several attempts at this for cultivated plants (Glen 2002) and trees (van Wyk & Glen 2016), but to create a full inventory that is kept regularly updated will be a major task made extremely difficult because many introduced plants are known only as cultivars by the nursery industry and many plants that have been introduced might no longer be cultivated. The scope of SAPIA should remain to record taxa growing outside of cultivation, though perhaps with the addition of a field for assessing whether populations are naturalised or invasive as per the Blackburn Scheme (see Wilson et al. 2014 for a field interpretation of the scheme for alien trees).
Even comparing lists of taxa in Henderson (2007) and this publication is complicated. In Henderson (2007), 601 taxa were listed but 44 of these, which were identified only to genus level, for example, Datura sp., but were most likely already listed, have been excluded from the current publication. Lemna gibba has been excluded because it is regarded as indigenous. Recent research has shown that Myriophyllum spicatum in South Africa should be regarded as indigenous (Weyl et al. 2016). Some species that appear on this list for the first time were previously known from the region but were misidentified, for example, H. balansae as Acanthocereus tetragonus, N. cordifolia as N. exaltata and F. foetida was confused with Agave sisalana. Such issues will likely continue in perpetuity, but it again highlights the need for careful documentation. Although it will not completely resolve this issue, the nomenclature used in SAPIA is now linked to that of the Botanical Research And Herbarium Management System (BRAHMS) and so ultimately to internationally agreed lists.
This publication only lists species alien to South Africa. Some extra-limital indigenous species and cultivars have been recorded in the SAPIA database but have been excluded from this publication, for example, Crocosmia cf. paniculata cultivar, Erica glandulosa, Euryops chrysanthemoides and Ipomoea cairica. Resolving issues of nativity below the level of a country is difficult though not intractable, and protocols are needed for dealing with such taxa when they spread or are spread beyond their natural distribution range and become problematic. SAPIA also contains some data of alien plants in neighbouring countries, but the level of sampling is much lower. Efforts are underway to develop SAPIA-type atlases for other parts of Africa (Arne Witt, CABI, pers. comm., July 2016). We hope that SAPIA can provide a framework for such initiatives, or at least serve as a practical example of the value of such data to research and management.
The quality and quantity of SAPIA data could be improved by having more data collectors country-wide and a central online facility for submitting and storing data. Plans to make all SAPIA data available online at the Weeds and Invasive Plants website (Henderson 2006) failed due to a complete breakdown in the management of the Agricultural Geo-Referenced Information System (AGIS) host site. Currently, the SAPIA database is housed at SANBI on the Pretoria server. A data-sharing agreement between ARC and SANBI has paved the way for the SAPIA data to soon become accessible through SANBI's BRAHMS online website.
Finally, the future of the SAPIA database is dependent on a secure source of funding. Funding over the past 16 years has been provided by the DEA, but this was only for coordination of the SAPIA project and roadside surveys by the lead author (L.H.). Much more funding is required for improvements to data collection, which will entail the employment of more dedicated data collectors, and for an online facility for storing and submitting data.
Conclusion
This review has highlighted rapidly spreading taxa, which require urgent attention. Some are already subjects of biological control programmes but should receive increased priority with the implementation of national species management programmes, for example, C. macrocephalum, P. hysterophorus, T. stans and O. engelmannii, whereas others should be considered for biological control, for example, C. grandiflora, G. triacanthos, P. setaceum and T. spachianus.
The small expansion and even contraction of some of the most prominent invaders such as Acacia longifolia, Acacia saligna, Acacia cyclops and A. filiculoides, which have been the subjects of successful biological control programmes, reinforces the value of biological control in the management of invasive alien species. However, the expansion of some species, despite the availability of effective biological control agents, such as C. jamacaru, Cylindropuntia imbricata and Opuntia stricta, indicates that there needs to be better implementation of biological control in some instances (Zachariades et al. 2017).
This review has shown that there is an ever-increasing number of invasive and potentially invasive taxa to deal with in South Africa, that is, there is a substantial invasion debt (Rouget et al. 2016). From our results, it is also clear that invasive plant taxa (particularly those that are listed in the regulations) are continuing to spread at alarming rates. More taxa should be considered for listing as invasive species under the NEM:BA A&IS Regulations; some taxa, which are mainly associated with disturbance and agricultural lands, should be removed from the NEM:BA A&IS Lists; proof of sterility needs to be obtained for the cultivars of 34 species which have been exempted; but ultimately more needs to be done to ensure that management is strategic and effective.
By enabling us to highlight these trends and issues, we believe SAPIA continues to be an essential tool for the monitoring and reporting on the status of alien plants in South Africa.
Acknowledgements
The authors would like thank all the people who have contributed in many different ways to the compilation of the SAPIA database. SAPIA is an initiative of the Agricultural Research Council (ARC). The South African National Biodiversity Institute (SANBI), where the lead author (L.H.) is stationed, is thanked for providing the office space and essential services for conducting this research.
ARC-Plant Protection Research Institute has provided the infrastructure, basal funding and support since the mid-1980s to the present. External funding of SAPIA has been gratefully received from the Department of Environmental Affairs - Natural Resource Management Programmes (NRMP).
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
L.H. initiated the manuscript, collated the data and led the writing. J.R.U.W. conducted the statistical analyses and helped with the writing.
References
Benjamini, Y. & Hochberg, Y., 1995, 'Controlling the false discovery rate - A practical and powerful approach to multiple testing', Journal of the Royal Statistical Society Series B - Methodological 57, 289-300. [ Links ]
Blackburn, T.M., Pyšek, P., Bacher, S., Carlton, J.T., Duncan, R.P., Jarošík, V. et al., 2011, 'A proposed unified framework for biological invasions', Trends in Ecology & Evolution 26, 333-339. https://doi.org/10.1016/j.tree.2011.03.023 [ Links ]
Breitwieser, I., Brownsey, P.J., Heenan, P.B., Nelson, W.A. & Wilton, A.D. (eds.), 2010-2016, Flora of New Zealand Online - Taxon Profile - Bartlettina sordida, viewed 14 July 2016, from http://www.nzflora.info/factsheet/Taxon/Bartlettina_sordida.html [ Links ]
Coetzee, J.A., Hill, M.P., Byrne, M.J. & Bownes, A., 2011, 'A review of the biological control programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S.Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides Lam. (Azollaceae) in South Africa', African Entomology 19, 451-468. https://doi.org/10.4001/003.019.0202 [ Links ]
Department of Environmental Affairs, 2014a, National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004) Alien and Invasive Species Lists, Government Gazette of South Africa, Pretoria, pp. 3-80. [ Links ]
Department of Environmental Affairs, 2014b, Government Notice R. 598, National Environmental Management: Biodiversity Act (10/2004): Alien and Invasive Species Regulations, Government Gazette No. 37885. [ Links ]
Faulkner, K.T., Hurley, B.P., Robertson, M.P., Rouget, M. & Wilson, J.R.U., 2017, 'The balance of trade in alien species between South Africa and the rest of Africa', Bothalia 47(2), a2157. https://doi.org/10.4102/abc.v47i2.2157 [ Links ]
Geerts, S., Botha, P.W., Visser, V., Richardson, D.M. & Wilson, J.R.U., 2013, 'Montpellier broom (Genista monspessulana) and Spanish broom (Spartium junceum) in South Africa: An assessment of invasiveness and options for management', South African Journal of Botany 87, 134-145. https://doi.org/10.1016/j.sajb.2013.03.019 [ Links ]
Germishuizen, G. & Meyer, N.L. (eds.), 2003, Plants of southern Africa: An annotated checklist, Strelitzia 14, National Botanical Institute, Pretoria. [ Links ]
Glen, H.F., 2002, Cultivated plants of Southern Africa, South African National Biodiversity Institute, Jacana, Johannesburg. [ Links ]
Greve, M., Mathakutha, R., Steyn, C. & Chown, S.L., 2017, 'Terrestrial invasions on Sub-Antarctic Marion and Prince Edward Islands', Bothalia 47(2), a2143. https://doi.org/10.4102/abc.v47i2.2143 [ Links ]
Henderson, L., 1989, 'Invasive alien woody plants of Natal and the north-eastern Orange Free State', Bothalia 19, 237-261. https://doi.org/10.4102/abc.v19i2.966 [ Links ]
Henderson, L., 1991a, 'Invasive alien woody plants of the Orange Free State', Bothalia 21, 73-89. https://doi.org/10.4102/abc.v21i1.868 [ Links ]
Henderson, L., 1991b, 'Invasive alien woody plants of the northern Cape', Bothalia 21, 177-189. https://doi.org/10.4102/abc.v21i2.885 [ Links ]
Henderson, L., 1992, 'Invasive alien woody plants of the eastern Cape', Bothalia 22, 119-143. https://doi.org/10.4102/abc.v22i1.830 [ Links ]
Henderson, L., 1998a, 'Southern African Plant Invaders Atlas (SAPIA)', Applied Plant Sciences 12, 31-32. [ Links ]
Henderson, L., 1998b, 'Invasive alien woody plants of the southern and southwestern Cape region, South Africa', Bothalia 28, 91-112. https://doi.org/10.4102/abc.v28i1.624 [ Links ]
Henderson, L., 2006, 'Plant Protection Research Institute initiatives: Southern African Plant Invaders Atlas (SAPIA) phase II', Plant Protection News 68, 5. [ Links ]
Henderson, L., 2007, 'Invasive, naturalized and casual alien plants in southern Africa: A summary based on the Southern African Plant Invaders Atlas (SAPIA)', Bothalia 37, 215-248. https://doi.org/10.4102/abc.v37i2.322 [ Links ]
Henderson, L., 2011, 'Mapping of invasive alien plants: The contribution of the Southern African Plant Invaders Atlas (SAPIA) to biological weed control', African Entomology 19, 498-503. https://doi.org/10.4001/003.019.0207 [ Links ]
Henderson, L. & Musil, K.J., 1984, 'Exotic woody plant invaders of the Transvaal', Bothalia 15, 297-313. https://doi.org/10.4102/abc.v15i1/2.1128 [ Links ]
Hill, M.P. & Coetzee, J.A., 2017, 'The biological control of aquatic weeds in South Africa: Current status and future challenges', Bothalia 47(2), a2152. https://doi.org/10.4102/abc.v47i2.2152 [ Links ]
Hulme, P.E., 2012, 'Weed risk assessment: A way forward or a waste of time?', Journal of Applied Ecology 49, 10-19. https://doi.org/10.1111/j.1365-2664.2011.02069.x [ Links ]
Impson, F.A.C., Kleinjan, C.A., Hoffmann, J.H., Post, J.A. & Wood, A.R., 2011, 'Biological control of Australian Acacia species and Paraserianthes lophantha (Willd.) Nielsen (Mimosaceae) in South Africa', African Entomology 19, 186-207. https://doi.org/10.4001/003.019.0210 [ Links ]
Jacobs, L.E.O., Richardson, D.M., Lepschi, B.P. & Wilson, J.R.U., 2017, 'Quantifying errors and omissions in the listing of alien species: Melaleuca in South Africa as a case-study', Neobiota 32, 89-105. https://doi.org/10.3897/neobiota.32.9842 [ Links ]
Kaplan, H., van Niekerk, A., Le Roux, J.J., Richardson, D.M. & Wilson, J.R.U., 2014, 'Incorporating risk mapping at multiple spatial scales into eradication management plans', Biological Invasions 16, 691-703. https://doi.org/10.1007/s10530-013-0611-z [ Links ]
Kaplan, H., Wilson, J.R.U., Klein, H., Henderson, L., Zimmermann, H.G., Manyama, P. et al., 2017, 'A proposed national strategic framework for the management of Cactaceae in South Africa', Bothalia 47(2), a2149. https://doi.org/10.4102/abc.v47i2.2149 [ Links ]
Klein, H., 2011, 'A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa', African Entomology 19, 515-549. https://doi.org/10.4001/003.019.0214 [ Links ]
Klein, H., 2012, 'Two biocontrol successes for the price of one', Plant Protection News 93, 1-2. [ Links ]
Latombe, G., Pyšek, P., Jeschke, J.M., Blackburn, T.M., Bacher, S., Capinha, C. et al., (in press), 'A vision for global monitoring of biological invasions', Biological Conservation. https://doi.org/10.1016/j.biocon.2016.06.013 [ Links ]
Le Maitre, D.C., Forsyth, G.G. & Wilson, J.R.U., 2015, Guidelines for the development of national species-based invasive alien management programmes: Setting geographically differentiated goals, Report No. CSIR/NRE/ECOS/ER/2015/0030/A, Natural Resources and the Environment, CSIR, Stellenbosch, p. 51. [ Links ]
McConnachie, A.J., Hill, M.P. & Byrne, M.J., 2004, 'Field assessment of a frond-feeding weevil, a successful biological control agent of red water fern, Azolla filiculoides, in Southern Africa', Biological Control 29, 326-331. https://doi.org/10.1016/j.biocontrol.2003.08.010 [ Links ]
McConnachie, A.J., Retief, E., Henderson, L. & Mc Kay, F., 2011, 'The initiation of a biological control programme against pompom weed, Campuloclinium macrocephalum (Less.) DC. (Asteraceae), in South Africa', African Entomology 19, 258-268. https://doi.org/10.4001/003.019.0217 [ Links ]
McGeoch, M.A., Genovesi, P., Bellingham, P.J., Costello, M.J., McGrannachan, C. & Sheppard, A., 2016, 'Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion', Biological Invasions 18, 299-314. https://doi.org/10.1007/s10530-015-1013-1 [ Links ]
Moran, V.C., Hoffmann, J.H. & Hill, M.P. (eds.), 2011a, 'Biological control of invasive alien plants in South Africa (1999-2010)', African Entomology (Special Issue) 19, 177-549. [ Links ]
Moran, V.C., Hoffmann, J.H. & Hill, M.P., 2011b, 'A context for the 2011 compilation of reviews on the biological control of invasive alien plants in South Africa', African Entomology 19, 177-185. https://doi.org/10.4001/003.019.0218 [ Links ]
Moran, V.C. & Zimmermann, H.G., 1991, 'Biological control of cactus weeds of minor importance in South Africa, Agriculture', Ecosystems and Environment 37, 29-35. https://doi.org/10.1016/0167-8809(91)90138-n [ Links ]
Novoa, A., Le Roux, J.J., Robertson, M.P., Wilson, J.R.U. & Richardson, D.M., 2015, 'Introduced and invasive cactus species - A global review', AoB Plants 7, plu078. https://doi.org/010.1093/aobpla/plu1078 [ Links ]
Parker, I.M., Simberloff, D., Lonsdale, W.M., Goodell, K., Wonham, M., Kareiva, P.M. et al., 1999, 'Impact: Toward a framework for understanding the ecological effects of invaders', Biological Invasions 1, 3-19. https://doi.org/10.1023/A:1010034312781 [ Links ]
POSA, 2012, Plants of Southern Africa: An online checklist, South African National Biodiversity Institute, viewed July 2016, from http://posa.sanbi.org [ Links ]
Pereira, H.M., Ferrier, S., Walters, M., Geller, G.N., Jongman, R.H.G., Scholes, R.J. et al., 2013, 'Essential biodiversity variables', Science 339, 277-278. https://doi.org/10.1126/science.1229931 [ Links ]
R Core Team, 2016, R: A language and environment for statistical computing, v. 3.3.2, R Foundation for Statistical Computing, Vienna, Austria. [ Links ]
Robertson, M.P., Cumming, G.S. & Erasmus, B.F.N., 2010, 'Getting the most out of atlas data', Diversity and Distributions 16, 363-375. https://doi.org/10.1111/j.1472-4642.2010.00639.x [ Links ]
Rouget, M., Richardson, D.M., Nel, J.L., Le Maitre, D.C., Egoh, B. & Mgidi, T., 2004, 'Mapping the potential ranges of major plant invaders in South Africa, Lesotho and Swaziland using climatic suitability', Diversity and Distributions 10, 475-484. https://doi.org/10.1111/j.1366-9516.2004.00118.x [ Links ]
Rouget, M., Robertson, M.P., Wilson, J.R.U., Hui, C., Essl, F., Rentería, J.L. et al., 2016, 'Invasion debt - Quantifying future biological invasions', Diversity and Distributions 22, 445-456. https://doi.org/10.1111/ddi.12408 [ Links ]
Silvertown, J., Harvey, M., Greenwood, R., Dodd, M., Rosewell, J., Rebelo, T. et al., 2015, 'Crowdsourcing the identification of organisms: A case-study of iSpot', Zookeys 480, 125-146. https://doi.org/10.3897/zookeys.480.8803 [ Links ]
Spies, J.J. & Du Plessis, H., 1987, 'Sterile Lantana: Fact or theory', South African Journal Plant & Soil 4,171-174. [ Links ]
Terblanche, C., Nänni, I., Kaplan, H., Strathie, L.W., McConnachie, A.J., Goodall, J. et al., 2016, 'An approach to the development of a national strategy for controlling invasive alien plant species: The case of Parthenium hysterophorus in South Africa', Bothalia: African Biodiversity and Conservation 46, a2053. https://doi.org/10.4102/abc.v46i1.2053 [ Links ]
The Plant List, 2013, Version 1.1, viewed July 2016, from http://www.theplantlist.org/ [ Links ]
US National Plant Germplasm System: GRIN Taxonomy, 2016, viewed July 2016, from https://npgsweb.ars-grin.gov/gringlobal/taxonomybrowse.aspx. [ Links ]
van Wilgen, B.W., Fill, J.M., Baard, J., Cheney, C., Forsyth, A.T. & Kraaij, T., 2016, 'Historical costs and projected future scenarios for the management of invasive alien plants in protected areas in the Cape Floristic Region', Biological Conservation 200, 168-177. https://doi.org/10.1016/j.biocon.2016.06.008 [ Links ]
van Wilgen, B.W., Forsyth, G.G., Le Maitre, D.C., Wannenburgh, A., Kotzé, J.D., van den Berg, E. et al., 2012, 'An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa', Biological Conservation 148, 28-38. https://doi.org/10.1016/j.biocon.2011.12.035 [ Links ]
van Wyk, B. & Glen, H., 2016, Guide to trees introduced into Southern Africa, Penguin Random House, Cape Town. [ Links ]
Venables, W.N. & Ripley, B.D., 2002, Modern applied statistics with S, 4th edn., Springer, New York. [ Links ]
Wells, M.J., Balsinhas, A.A., Joffe, H., Engelbrecht, V.M., Harding, G. & Stirton, C.H., 1986, A catalogue of problem plants in southern Africa, Memoirs of the Botanical Survey of South Africa No. 53., Department of Agriculture and Water Supply. [ Links ]
Wells, M.J., Duggan, K.J. & Henderson, L., 1980, 'Woody plant invaders of the central Transvaal', Proceedings of the third National Weeds Conference of South Africa, p. 11-23, 1979, Balkema, Cape Town. [ Links ]
Weyl, P.S.R., Thum, R.A., Moody, M.L., Newman, R.M. & Coetzee, J.A., 2016, 'Was Myriophyllum spicatum L. (Haloragaceae) recently introduced to South Africa from Eurasia?', Aquatic Botany 128, 7-12. https://doi.org/10.1016/j.aquabot.2015.09.003 [ Links ]
Wilson, J.R.U., Gaertner, M., Richardson, D.M. & van Wilgen, B.W., 2017a,' Contributions to the National Status Report on Biological Invasions in South Africa', Bothalia 47(2), a2207. https://doi.org/10.4102/abc.v47i2.2207 [ Links ]
Wilson, J.R., Panetta, F.D. & Lindgren, C., 2017b, Detecting and responding to alien plant incursions, Cambridge University Press, Cambridge, U.K. p. 286. [ Links ]
Wilson, J.R.U., Caplat, P., Dickie, I., Hui, C., Maxwell, B.D., Nuñez, M.A. et al., 2014, 'A standardized set of metrics to assess and monitor tree invasions', Biological Invasions 16, 535-551. https://doi.org/10.1007/s10530-013-0605-x [ Links ]
Wilson, J.R.U., Ivey, P., Manyama, P. & Nänni, I., 2013, 'A new national unit for invasive species detection, assessment and eradication planning', South African Journal of Science 109(5/6), Art. #0111, 1-13. https://doi.org/10.1590/sajs.2013/20120111 [ Links ]
Wilson, J.R.U., Richardson, D.M., Rouget, M., Procheş, Ş., Amis, M.A., Henderson, L. et al., 2007, 'Residence time and potential range: Crucial considerations in modelling plant invasions', Diversity and Distributions 13, 11-22. https://doi.org/10.1111/j.1366-9516.2006.00302.x [ Links ]
Wood, A.R., 2017, 'Fungi and invasions in South Africa', Bothalia: African Biodiversity and Conservation, Special Issue, tbc. [ Links ]
Zachariades, C., Hoffmann, J.H. & Roberts, A.P., 2011, 'Biological control of mesquite (Prosopis species) (Fabaceae) in South Africa', African Entomology 19, 402-415. https://doi.org/10.4001/003.019.0230 [ Links ]
Zachariades, C., Paterson, I.D., Strathie, L.W., Hill, M.P. & van Wilgen, B.W., 2017, 'Assessing the status of biological control as a management tool for suppression of invasive alien plants in South Africa', Bothalia: African Biodiversity and Conservation, Special Issue, tbc. [ Links ]
Zengeya, T., Ivey, P., Woodford, D.J., Weyl, O., Novoa, A., Shackleton, R. et al., 2017, 'Managing conflict-generating invasive species in South Africa: Challenges and trade-offs', Bothalia: African Biodiversity and Conservation, Special Issue, tbc. [ Links ]
 Correspondence:
Correspondence:
Lesley Henderson
l.henderson@sanbi.org.za
Received: 06 Sept. 2016
Accepted: 23 Feb. 2017
Published: 31 Mar. 2017
Note: This paper was initially delivered at the 43rd Annual Research Symposium on the Management of Biological Invasions in South Africa, Goudini Spa, Western Cape, South Africa on 18-20 May 2016.
Details of categorisation schemes used to classify alien plants.
References
Klein, H., 2011, A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa, African Entomology 19, 515-549. [ Links ]
Moran, V.C., Hoffmann, J.H. & Hill, M.P., 2011, A context for the 2011 compilation of reviews on the biological control of invasive alien plants in South Africa, African Entomology 19, 177-185. [ Links ]
References
Department of Environmental Affairs, 2014a, National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004) Alien and Invasive Species Lists, Government Gazette of South Africa, Pretoria, pp. 3-80. [ Links ]
Department of Environmental Affairs, 2014b, Government Notice R. 598, National Environmental Management: Biodiversity Act (10/2004): Alien and Invasive Species Regulations, Government Gazette No. 37885. [ Links ]

FIGURE 1-A4 - Click to enlarge
References
Blackburn, T.M., Pyšek, P., Bacher, S., Carlton, J.T., Duncan, R.P., Jarošík, V., Wilson, J.R.U. & Richardson, D.M., 2011, A proposed unified framework for biological invasions, Trends in Ecology & Evolution 26, 333-339. [ Links ]
Jacobs, L.E.O., Richardson, D.M., Lepschi, B.P. & Wilson, J.R.U., 2017, Quantifying errors and omissions in the listing of alien species: Melaleuca in South Africa as a case-study, Neobiota 32, 89-105. [ Links ]
Magona, N. unpublished data.
Statistical models of the increase in recorded distribution of alien plants in SAPIA between 2000 and 2016 as a function of their distribution in 2000.
a) The first model includes the full data set
Call: glm.nb (formula = increase in number of qds occupied 2000 to 2016 ~ log(distribution in 2000 + 1), init.theta = 0.713, link = log).
Dispersion parameter for Negative Binomial (0.7321) family taken to be 1.
Null deviance: 1373 on 771 degrees of freedom.
Residual deviance: 857 on 770 degrees of freedom.
AIC: 4675.1.
Number of Fisher Scoring iterations: 1.
Theta: 0.7312
Std. Err.: 0.0403
2 x log-likelihood: -4669
Plotted out this looks like
b) The second model is restricted to only those taxa which are known not to be systematically under-reported in SAPIA, and is plotted in the main paper as Figure 2. Notably removing taxa that were known to previously have been under-reported improved the fit of the model (cf. dispersion parameters and deviance residuals).
Call: glm.nb(formula = increase in number of qds occupied 2000 to 2016 ~ log(distribution in 2000 + 1), init.theta = 0.852, link = log).
Dispersion parameter for Negative Binomial (0.915) family taken to be 1.
Null deviance: 1212.45 on 564 degrees of freedom.
Residual deviance: 622.7 on 563 degrees of freedom.
AIC: 3416.6.
Number of Fisher Scoring iterations: 1.
Theta: 0.915.
Std. Err.: 0.0634.
2 x log-likelihood: -3410.
c) The final model including the influence of regulatory status (for a description of the regulatory categories see Appendix 2c).
Call: glm.nb(formula = increase in number of qds occupied 2000 to 2016 ~ log(distribution in 2000 + 1) + NEM:BA A&IS category, init.theta = 0.852, link = log)
Dispersion parameter for Negative Binomial (1.0458) family taken to be 1
Null deviance: 1352.26 on 564 degrees of freedom
Residual deviance: 612.25 on 559 degrees of freedom
AIC: 3354.6
Number of Fisher Scoring iterations: 1
Theta: 1.046
Std. Err.: 0.0744
2 x log-likelihood: -3341