Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Bothalia - African Biodiversity & Conservation
On-line version ISSN 2311-9284
Print version ISSN 0006-8241
Bothalia (Online) vol.46 n.2 Pretoria 2016
http://dx.doi.org/10.4102/abc.v46i2.2110
ORIGINAL RESEARCH
A lot gone but still hanging on: Floristics of remnant patches of endangered KwaZulu-Natal Sandstone Sourveld
Charmaine C. DruryI; Syd RamdhaniI; Sershen NaidooI; Clinton CarbuttII; Renira BoodhrajI; Philani MbathaI
ISchool of Life Sciences, Westville Campus, University of KwaZulu-Natal, South Africa
IISchool of Life Sciences, Pietermaritzburg Campus, University of KwaZulu-Natal, South Africa
ABSTRACT
BACKGROUND: KwaZulu-Natal Sandstone Sourveld (KZNSS) is an endangered subtropical grassland type, of which a large proportion occurs within the eThekwini Municipal Area (EMA).
OBJECTIVES: Examining the flora of KZNSS will allow a more fundamental understanding of the potential variability across remnant patches of this vegetation type, increasing the ability to accurately delimit KZNSS from adjacent similar vegetation types.
METHOD: Floristic data were collected using quadrats and transects for three recognised KZNSS sites (Giba Gorge Environmental Precinct (GGEP), Inanda Mountain (IM) and Springside Nature Reserve (SSNR)), all within the EMA. Alpha diversity (Shannon's exponential and Simpson's inverse indices) and beta diversity measures were calculated and compared across all sites. An unweighted pair group method with arithmetic mean (UPGMA) analysis using the Jaccard index and a non-parametric multidimensional scaling (NMDS) ordination were used to assess similarity amongst quadrats across the three sites.
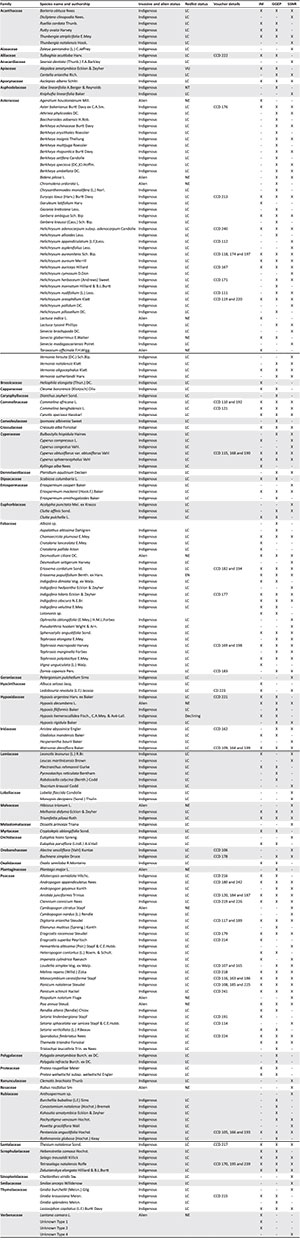
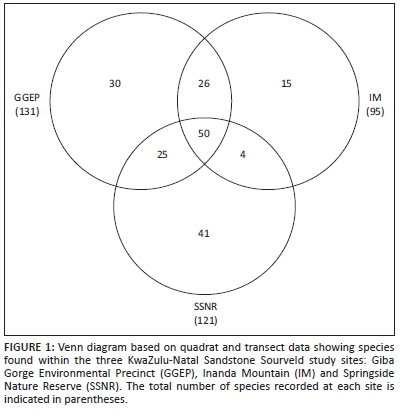
RESULTS: One hundred and thirty-one plant species were found to occur in GGEP, 95 in IM and 121 in SSNR. However, of the total 193 species found to occur collectively (i.e. quadrat and transect data combined) across the three sites, only 50 species were common to all these sites. The results of the alpha and beta diversity analyses revealed significant floristic variability both within and across the KZNSS sites sampled, with Shannon's exponential index being highest in SSNR, followed by GGEP and lowest in IM. The lack of controlled access and unregulated burning regimes appear to have clearly affected the flora at the IM site in terms of species richness and increased evenness, as well as the relatively greater presence of introduced alien species and lower abundances of taxa of conservation concern. The pristine GGEP site had the highest number of species in total, with species being less evenly spread across the site, as well as the highest number of taxa conservation and low abundances of alien species. The main separations in the ordination results can be attributed to quadrat sampling performed pre- and post-burn.
CONCLUSION: The floristic distinction of IM from GGEP and SSNR is attributed here to the intermediate disturbance effect of fire in grasslands which can lead to species loss if burning is too frequent. The implications of these findings are discussed in the context of the delimitation, classification and management of KZNSS.
Introduction
Subtropical grasslands in South Africa are found mostly along the eastern seaboard, in the Maputaland-Pondoland-Albany biodiversity hotspot, where suitable climate prevails. However, this biodiversity hotspot has a very high overall vulnerability, being threatened by invasive alien plant species (IAPS), urbanisation and agricultural activities (Jonas et al. 2006). Rouget et al. (2006) found that many of the vegetation types (including grasslands) within this hotspot are unique in terms of floristic composition, yet are subject to very little protection, with less than 60% of their natural habitat remaining. Protection levels are poorest around urban areas, where the overall vulnerability is higher than the surrounding rural areas (Jonas et al. 2006).
One of the most severely transformed vegetation types in the region is KwaZulu-Natal Sandstone Sourveld (KZNSS). A significant part of the range of KZNSS is found within the eThekwini Municipal Area (EMA) within KwaZulu-Natal Province. This vegetation type, located within an urban matrix, is listed by the Department of Environmental Affairs (2011) as endangered owing to the irreversible loss of natural habitat. KZNSS falls within the Savanna biome and was coded as SVs5 by Rutherford et al. (2006). New patches of KZNSS are being identified and, with the present study, further increases in the size of KZNSS are likely. It is a species-rich grassland type with high levels of endemism, characterised by scattered geoxylic suffrutices, low shrubs and proteas occurring on flat or rolling plateau tops and steep slopes. According to Rutherford et al. (2006), KZNSS contains 12 endemic taxa (i.e. diagnostic taxa), namely Brachystelma modestum R.A.Dyer, B. natalense (Schltr.) N.E.Br., B. pulchellum (Harv.) Schltr., Crassula inandensis Schönland & Baker f., Cynorkis compacta (Rchb.f.) Rolfe, Eriosema populifolium subsp. populifolium Benth. ex Harv., E. rossii (Oliv.) Källersjö, Gladiolus cruentus T.Moore, Helichrysum woodii N.E.Br., Hesperantha gracilis Baker, Phymaspermum pinnatifidum (Oliv.) Källersjö and Tephrosia inandensis H.M.L.Forbes. However, some of these endemics are confined to sandstone cliffs rather than the flatter grassland portion of KZNSS (e.g. Crassula inandensis, Cynorkis compacta, Gladiolus cruentus, Hesperantha gracilis). This list of endemic taxa is based on expert opinion which may not be all inclusive, given that approximations were necessary. Data generated by studies such as the present one should be used to update existing lists.
KZNSS occurs along an altitudinal range of 500 - 1100 metres above sea level (m.a.s.l.) in the summer rainfall region that experiences mist, which is important in providing additional moisture (Table 1). Frost is a very rare occurrence within the region (Rutherford et al. 2006). Similar vegetation types occurring within the EMA include Ngongoni Veld (SVs4) and KwaZulu-Natal Coastal Belt Grassland (CB3), both of which experience similar environmental conditions and contain many of the same plant species as KZNSS (Rutherford et al. 2006). Again these overlaps, particularly in terms of species, are based on expert opinion. However, CB3 is more similar to KZNSS than SVs4 in terms of environmental parameters (Table 1; Mucina & Rutherford 2006). Although these other vegetation types were not directly examined in the present study, they may offer insights into KZNSS owing to their geographic proximity and similarity to KZNSS (Table 1).
KZNSS grasslands within the EMA have experienced extensive transformation primarily through urbanisation, resulting in small isolated fragments that are often further compromised by IAPS and unmanaged fire regimes (Department of Environmental Affairs 2011). The floristic variability and degree of transformation across patches is, however, heterogeneous and needs to be examined more closely to determine the effects that various anthropogenic activities, associated abiotic and biotic changes and management practices might have had on the floristic integrity of KZNSS. Parr et al. (2014) noted that '… underpinning all of the threats facing tropical grassland biomes is the misclassification of vegetation that results in inappropriate management techniques'. Given the geographical and environmental proximity between KZNSS and similar vegetation types (Table 1), remnant patches of KZNSS must be carefully floristically profiled for proper classification. On this note, KZNSS has a history of being grouped with other similar vegetation types in the area, for which it is easily mistaken, and has only recently being separated from some of them by Mucina and Rutherford (2006). Vegetation delimitation is scale dependent, and vegetation units are not expected to have exactly the same floristic composition or signature. Additionally, within vegetation types are nested plant communities that have a similar floristic composition and structure (e.g. rocky outcrops) that are often grouped into the broader and/or different vegetation types. Delimiting KZNSS is a problem experienced by land owners, the local/municipal conservation bodies and other stake holders that are tasked with KZNSS conservation. Given the vague delimitation of KNZSS, its high conservation priority and the close similarity to other grasslands types (in terms of distribution, floristics and environmental variables) (Table 1), remnant patches of KZNSS must be examined more carefully to identify conservation priorities.
The present study therefore aimed to understand the floristic variability within and across three KZNSS grassland fragments with a view to developing a floristic signature and characterisation that can help delimit KZNSS, and distinguish it floristically from similar subtropical grassland types.
Methods and materials
Study sites
Three KZNSS sites were selected for investigation after consultation with local botanists and confirmatory field visits. These included Inanda Mountain (IM), Giba Gorge Environmental Precinct (GGEP) and Springside Nature Reserve (SSNR). Some environmental characteristics associated with each of the sites, extracted from the broader literature on KZNSS, are shown in Table 2.
Data collection
At each of the three sites, a minimum of fifteen 5 × 5 m quadrats were laid out as prescribed by Curtis and Cottom (1956) and spaced between 5 m and 30 m apart, depending on the site, to accommodate potential variation across the site, including aspect and slope, but steep cliffs were excluded. Sampling only included the grassland portions of each site. Species abundance was determined within each quadrat and voucher specimens of each species were collected for taxonomic identification to species, and in some cases to subspecies level. For logistic reasons and to accommodate for seasonality, c. 60% of the quadrats at all sites were surveyed during winter (1 June - 31 August, 2012-2014), an additional c. 30% during summer (1 December - 28 February, 2012-2014). The remaining c. 10% of quadrats were performed during autumn (1 March - 31 May, 2013-2014) and spring (1 September -30 November, 2012-2014).
Walked diagonal transects were also undertaken at each site, once a month, across all seasons to collect species in flower that were previously unrecorded in the quadrats. This helped to more accurately inform the presence of species at each site so that objective comparisons could be made and comprehensive floristic profiles could be generated for each site. All specimens in flower were collected and deposited at the Ward Herbarium (UDW), Westville Campus, University of KwaZulu-Natal, Durban, South Africa.
Data analysis
EstimateS 9.0 (Colwell 2013) was used to construct species accumulation curves for the quadrat data only, to determine if sufficient sampling had been performed. The non-parametric estimators, Chao2 and Jack1, were used to estimate total species richness. Owing to time constraints, it was not possible to conduct quadrat sampling to the point where all species, especially rare species, were encountered. Percentage sampling effort was therefore calculated by dividing the number of species found by the projected number of species using the Jack1 and Chao2 estimators. This process was repeated after every five quadrats at each site were sampled. Sampling effort ceased when the average percentage sampling effort (using Jack1 and Chao2 estimators) reached an acceptable value (≥ 80%).
EstimateS 9.0 (Colwell 2013) was also used to calculate the Simpson's inverse and Shannon's exponential indices to compare alpha diversities of the sites based on quadrat data. Beta diversities were examined in terms of species richness (using the measurement of βgl) and species turnover (using β-3 as a narrow-sense measure of species turnover as well as βt as a broad-sense measure of species turnover) (see Koleff, Gaston & Lennon 2003). All beta diversity measurements (βgl, β-3 and βt) were performed using all quadrats at each site and quadrats were also compared within sites. The resulting beta diversity measurements for the average values within each site were then compared by using analysis of variance (ANOVA) in SPSS version 22. Only βgl was found to be parametric (by a Shapiro-Wilks test; p > 0.05) with the assumption of homogeneity of variance supported (by Levene's test; p > 0.05) and significantly different (p < 0.05). Cluster analyses were performed using the presence/absence data of species in quadrats across the three sites. The add-on package of BiodiversityR (Kindt & Coe 2005) was used in conjunction with R (R Core Team 2016) to generate a similarity matrix using the Jaccard index, and clustering was achieved using the unweighted pair group method with arithmetic mean (UPGMA). Thereafter BiodiversityR was used to generate a Bray-Curtis similarity matrix and a non-parametric multidimensional scaling analysis (100 runs) was performed. The groups identified by the UPGMA were combined with the ordination plot to gain a better understanding of the data and the influences of vegetation structure as well as composition on the grouping of quadrats (after Pinto et al. 2013).
Results
The three sites range in altitude with IM (lowest point 428 m.a.s.l) being the lowest and SSNR (highest point 652 m.a.s.l.) being the highest above sea level (Table 2). Additionally, IM is the most northern and eastern site whilst GGEP is the most southern site, and SSNR is the most western site; SSNR and GGEP are closer to each other than either of them are to IM in terms of size, altitude and geographic location (Table 2). Whilst GGEP and SSNR experience controlled burning and are subject to controlled access, as both sites are under eThekwini Municipality management, IM is on communal land that experiences uncontrolled burning and is not protected in terms of controlled access (Table 2). The socio-economic status and land-use patterns of the areas in which the sites are situated differ as well; whilst GGEP and SSNR are located in suburban areas (Winston Park and Hillcrest, respectively), IM is situated in a rural part of the Inanda-Ntuzuma-KwaMashu area (Table 2).
Cumulatively, a total of 193 plant species were found at the three sites; however, only 50 of these were common to all three sites (for details, see Appendix 1). Figure 1 shows that GGEP had the highest number of total and unique species, followed by SSNR and then IM. At both IM and SSNR, 18 quadrats were required to reach an 80% sampling effort, whilst at GGEP 20 quadrats achieved an 80% sampling effort. Eighteen quadrats accounted for 74 and 111 species at IM and SSNR, respectively, while 20 quadrats accounted for 98 species at GGEP. Transect sampling increased the total number of species from 74 to 95, 111 to 121, and 98 to 131 for IM, SSNR and GGEP, respectively. Plant families most well represented at the three sites (i.e. quadrat and transect data combined) were Asteraceae, Poaceae and Fabaceae, with 44, 28 and 24 species, respectively (Appendix 1). These three families contributed to 50%of the total species found across the three sites.
Several taxa identified to be of conservation concern on the Red List of South African Plants (South African National Biodiversity Institute (SANBI) 2015) were found at study sites. These included, Hypoxis hemerocallidea Fisch, C.A. Mey. & Avé-Lall which is declining, Aloe linearifloia A.Berger & Reynolds which is near-threatened, Alepidea amatymbica Ecklon & Zeyher which is vulnerable, and Eriosema populifolium subsp. populifoilum Benth. ex Harv. which is endangered. Of the 12 species known to be diagnostic of (i.e. endemic to) KZNSS, only 1 was found, namely Eriosema populifolium subsp. populifolium Benth. ex Harv., which occurred at all three sites. Three Category 1b IAPS (species which should be removed immediately as per the Department of Environmental Affairs (DEA) 2014) were found (Table 3): Lantana camara L. was in low abundance at IM; Chromolaena odorata L. was in low abundances at GGEP and SSNR but abundant in IM; and Ageratum houstonium Mill. was abundant in IM. Additionally, 7 naturalised exotic species (3 at GGEP, 4 at IM, and 5 at SSNR) as well as 4 non-native species (2 at GGEP, 1 at IM, and 3 at SSNR) were found (Table 3).
The highest value for Shannon's exponential index was found for SSNR, whilst the Simpson's inverse index was highest for GGEP (Table 4). IM had the lowest values for both these alpha diversity indices (Figure 1 and Table 4). The Shannon's exponential index places emphasis on species richness whilst the Simpson's inverse index is known to give an estimate that emphasises evenness (Luis 1996; Nagendra 2002). Shannon's exponential index was highest for SSNR which also had the highest species richness of 111 based on quadrat sampling and increased to 121 species with transect sampling. Interestingly, GGEP had 98 species with quadrat sampling, which increased to 131 species with transect sampling, giving it the highest 'cumulative' species richness. IM had the lowest Shannon's exponential index value, explained by its low species richness for both quadrat (74) and transect (95) sampling. The Simpson's inverse index reflects higher evenness at lower values for this index (Luis 1996); IM had the lowest Simpson's inverse index, with higher values being recorded for SSNR and GGEP, respectively.
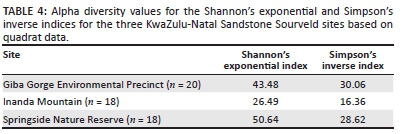
Three beta diversity analyses (βgl, β-3 and βt) were used to compare quadrats within each site using presence-absence of species in each quadrat. Only βgl, which is a measure of species richness gradients, was found to be significantly (p < 0.05) different across sites (Figure 2) with GGEP (which had the highest cumulative number of species) having the highest value, followed by IM and then SSNR (Figure 1).
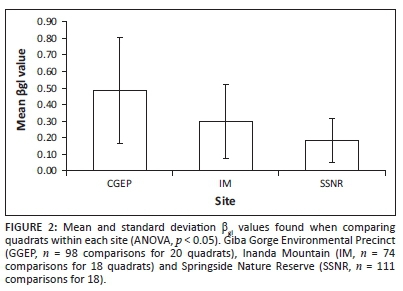
The species richness gradient beta diversity measure, βgl, allows insights into the spread of species and species evenness (Koleff, Gaston & Lennon 2003). This measure has a minimum value of 0, indicating complete similarity and a maximum value of 2, indicating complete dissimilarity in species composition (Koleff, Gaston & Lennon 2003). The beta diversity results show that all sites have a fairly low richness gradient. SSNR had the lowest βgl value (Figure 2) (i.e. quadrats reflect highest similarity in terms of species and evenness) and the highest value for Shannon's exponential index (Table 4), and second highest number of species in total (Figure 1). However, it should be noted that this number is only ten lower than GGEP, which had the highest cumulative number of species. Additionally, IM has a slightly higher species richness gradient (Figure 2), as well as the lowest number of species, which is supported by Shannon's exponential index (Figure 1 and Table 4), indicating that quadrats in the site are fairly similar and species are evenly distributed across the site. GGEP had the highest βgl value (Figure 2) and thus the least similarity in terms of quadrats and evenness of species, which is supported by the high Simpson's inverse index value (Table 4). However, this resultmay well be because of this site having the highest cumulative number of species with many less abundant species (see Luis 1996) (Figure 1), or alternatively because of temporal variations as a result of sampling occurring over different seasons.
The four clusters of the phenogram generated using UPGMA (not shown) were largely congruent with the NMDS results (Figure 3). Consequently, only the NMDS plot is shown in Figure 3. Alphabets (A-D) indicate the four clusters of quadrats in the UPGMA, and the number of quadrats from the respective sites are given below: A (GGEP = 0, IM = 3 and SSNR = 2), B (GGEP = 10, IM = 6 and SSNR = 0), C (GGEP = 10, IM = 9 and SSNR = 0) and D (GGEP = 0, IM = 0 and SSNR = 16). On the NMDS plot (Figure 3), it can also be seen that Clusters B and C are clearly distinct, whilst Clusters A and D are largely distinct, except for two quadrats from Cluster A that are close to those from Cluster D.
Discussion
Both GGEP and SSNR fall within the altitudinal ranges described for KZNSS (Tables 1 and 2). However, IM's highest point (476 m.a.s.l.) is lower than the 500-1100 m.a.s.l. typically associated with KZNSS. However, the altitude can extend up to 800 m in some parts of IM. The underlying geology of all three sites is Ordovician Natal Group Sandstone (Bell & Lindsay 1999; Table 1). The IM site is affected by a variety of factors, most importantly the lack of controlled access and formal management which gives rise to many forms of disturbance. The IM site is also used as a thoroughfare, resulting in high levels of litter, is used for low intensity grazing, and experiences burns (usually in early autumn) (author's personal observations, 2012-2015) which are uncontrolled (Table 2).
Of the three sites examined, GGEP had the highest cumulative (i.e. quadrats and transect data combined) number of species (n = 131; Figure 1) whilst also containing all 4 of the species of conservation concern found across the three sites (Table 3). This site is monitored and managed by the eThekwini Municipality and is therefore less prone to degradation and disturbance(s). With only a slightly lower cumulative number of species than GGEP, SSNR (n = 121, Figure 1) is also managed by the eThekwini Municipality and has more unique species than GGEP (Figure 1), which may be a consequence of its larger area than GGEP (Table 2). Based on the area effect of island biogeography theory, IM would therefore be expected to have the highest number of species (e.g. Bond, Midgley & Vlok 1988), but this was not the case. In fact, despite being the largest site (Table 2), IM exhibited the lowest cumulative number of species (n = 95) across the three sites. Additionally, it is worth mentioning that geoxylic taxa (a characteristic feature of KNZSS) are well adapted to fire and persist underground for long periods of time (Maurin et al., 2014), and many of these might have been undetected in the standing flora of the present study. Bond, Midgley & Vlok (1988) found that fire management plays a large role in determining species richness of islands in the fire-dependent fynbos ecosystem; similarly, improper fire management coupled with degradation may be responsible for the deviation from island biogeography theory observed at IM. Fynn, Morris & Edwards (2004) found that annual burns, when combined with grazing, resulted in a decrease of forb species richness whilst grass species richness remained the same as compared with biennial burns, where burning occurs in winter. This finding may explain the deviation of IM from the area effect of island biogeography theory, with the lower species richness possibly being a consequence of annual as opposed to biennial burns as well as disturbance caused by grazing and footpaths at this site. The three most dominant families across the three sites were Asteraceae, Poaceae and Fabaceae which were found to contribute to 50% of the total species found. However, in a grassland study further inland in the Platberg region of the eastern Free State (in South Africa), the four most dominant families in decreasing order were Asteraceae, Poaceae, Cyperaceae and Fabaceae, and these four families accounted for only 40.4% of the 441 taxa found in the study region (Brand, Brown & du Preez 2010). A possible reason for the differences in dominant families, particularly Cyperaceae, between the present study and that of Brand, Brown & du Preez (2010) is that, whilst the present study was performed purely within the grassland sections of each site, Brand Brown & du Preez (2010) included wetlands which are expected to contain more Cyperaceae species.
All of the 4 taxa of conservation concern were found at GGEP whilst only 1 was found at SSNR and 3 at IM (Table 3). This is not congruent with the species richness nor size trends across sites, and could be as a result of historical differences across sites or the influence of adjacent vegetation types that may house these taxa. Interestingly, 6 of the 14 alien species found in this study were mostly rare (i.e. less than 5 individuals observed per 100 m2) at GGEP, 9 were rare to very rare (i.e. one individual observed per 100 m2) at SSNR, and 9 were rare to common (i.e. 10 - 50 individuals observed per 100 m2) at IM (Table 3). This finding suggests that the number of alien taxa found at a site may not be a proper reflection of the abundance of aliens at the sites or that the management at sites is sufficient to suppress alien plant infestations at sites where there are higher numbers of alien taxa, but in lower abundances (e.g. SSNR in this study). At IM, where there is uncontrolled access and annual burning (Table 2), all three of the Category 1b IAPS found were present, 2 of which were common at the site (Table 3). At GGEP and SSNR, where there is controlled access and biennial burning (Table 2), only 1 of the 3 Category 1b IAPS (viz., Chromolaena odorata L.) was found and its occurrence was rare (Table 3). Additionally, the other alien taxa at GGEP and SSNR were mostly rare to very rare at all these sites (i.e. 1 - 5 individuals observed per 100 m2) (Table 3). These data suggest that the lack of appropriate management practices and relatively higher vulnerability to disturbances at KZNSS sites (as at IM) may not necessarily lead to increased alien species diversity, but can favour higher abundances of fewer existing alien species. As mentioned above, some endemics are confined to the cliffs. Despite this, only 1 of the 12 endemic KZNSS taxa was found in this study, which could be a reflection of the high levels of transformation that KZNSS patches have been subjected to over the years, as a consequence of anthropogenic pressures associated with urbanisation. The results of the present study further validate reports by SANBI that most of these endemic KZNSS taxa are rare or vulnerable (Source: http://redlist.sanbi.org/).
The alpha diversity results (Table 4) suggest that there may be more species that were not found in SSNR when compared with GGEP during the present study, as shown by the increase in the number of species when transect sampling is included. Additionally, factors such as quadrat placement and micro-environmental site differences could have yielded this result, despite careful quadrat and micro-environmental considerations. The IM site had the lowest number of species which are fairly evenly spread over the site. However, Luis (1996) noted that this evenness index (Simpson's inverse index) focuses more on abundant species; in the case of the present study, the most abundant species were grasses, suggesting that IM's grass species are evenly distributed across the site. For SSNR and GGEP, alpha diversity results can be explained in terms of the area effect of island biogeography as SSNR is larger in area than GGEP; but yet again, in contradiction of the effect, the largest site, of IM, exhibited the lowest richness. When the sites are compared in terms of the distance effect, the results still do not conform to what would be expected. As alluded to earlier, the poor (lack of) management at IM and its relatively higher vulnerability to disturbance may explain why it displayed the highest evenness and lowest species richness. Controlled fire (which is a disturbance) promotes seed germination in some species and this can be good for seedling recruitment (which could be the case at GGEP and SSNR); however, uncontrolled fires can damage sensitive seeds and reduce the opportunity for seed bank accumulation (Benson 1985). Therefore, sites with regular fire occurrences are likely to have lower levels of seedling recruitment and hence a reduction in species richness and increased evenness, which might have been the case for IM.
Beta diversity analyses support earlier indications that IM species were spread evenly across the site. Additionally, beta diversity analyses indicated that GGEP had the least even spread of species, which means that quadrats at GGEP (which exhibited the highest number of species) are likely to be less similar to each other in terms of species composition than those at IM. This observation suggests that the poor management practices at IM might have also led to increased homogenisation of the remaining vegetation via the reduction of species in the germinable soil seed bank (Benson 1985).
Disturbances have been reported to lead to ecosystem degradation in a number of vegetation types (e.g. Brand, Brown & du Preez 2010; Jonas et al. 2006) and the open access, disturbances and improper burning appear to be leading to biodiversity loss and increased levels of species homogenisation at the IM site. In total, there were 45 families at all 3 sites (29, 33 and 33 at IM, GGEP and SSNR, respectively), with 64%, 73% and 73% of the total number of families being present in IM, GGEP and SSNR, respectively. Furthermore, of the 129 genera found in the study, 74, 92 and 85 were present at IM, GGEP and SSNR, respectively. This implies that IM, GGEP and SSNR had 57%, 71% and 66% generic representation, respectively. We also examined the ratio of grasses species (Poaceae) to species from all other families to check if IM differed from the other two sites in this aspect. The ratio of grass species to other species was 19:76 (1:4,0), 20:111 (1:5,6) and 20:101 (1:5,1) at IM, GGEP and SSNR, respectively (or 20%, 15% and 17%, respectively). This implies that IM had a slightly higher percentage of grass representation overall. The data described above further support the possibility of homogenisation at IM. However, it is encouraging to note that this degradation did not result in significant separation of most of the IM quadrats from those of the other two sites (Figure 3), and that 3 of the 4 taxa of conservation concern found in this study were present at IM (Table 3). The minor contradictions to this assertion shown in Figure 3 may also be explained by the sampling strategy adopted in the present study and differences in the timing and frequency of burning carried out at the three sites, as opposed to actual floristic differences. For example, burning at IM took place not only more frequently but also earlier, in autumn, as opposed to the early winter burns carried out biannually at GGEP and SSNR (Table 2).
Quadrats of Cluster A were from SSNR and IM and sampled post-burning, and only quadrats of IM in Cluster A separated out distinctly in the ordination. Quadrats of Cluster B were from IM and GGEP and were sampled pre-burning. Quadrats from Cluster C were sampled pre-burning from IM, as well as sampled both pre-and post-burning at GGEP. Lastly, quadrats from Cluster D were from SSNR, and sampled both pre-and post-burning. Furthermore, we noticed over the period 2012 to 2015 that IM was burnt annually in April or early to mid-May during the three years over which the present study was conducted, whilst GGEP and SSNR were only burnt in June 2014. The seasonality and frequency of burning influences the timing, type and abundance of species that emerge (and subsequently establish) in the season following a burn (Benson 1985). Furthermore, IM contained the least number of unique and cumulative number of species (Figure 1) and post-burn IM quadrats were floristically distinct from all other quadrats, including those of pre-burn IM, pre- and post-burn SSNR as well as GGEP (Figure 3). The intermediate disturbance effects of fire in grasslands has revealed that annual burns can reduce the number of species at a particular site to a subset of the species found in similar sites where burning is less frequent (Collins, Glenn & Gibson 1995). The intermediate disturbance effect could therefore explain the results obtained here.
Additionally, IM, unlike the other two sites, is subject to regular grazing pressure (Table 2) and uncontrolled grazing can lead to altered species composition in grasslands (Fynn, Morris & Edwards 2004). Grazing occurs at IM and, to better facilitate this, fire is used in early autumn to encourage a flush of palatable grasses for cattle and goat livestock. The effects of burning time combined with simulated grazing in the long term (mowing in late summer and burning in winter) resulted in a 27% - 42% decrease in species richness in Southern Tall Grassveld at Ukulinga (Fynn, Morris & Edwards 2004). However, we cannot comment any further regarding how grazing and burning could have influenced the results of this study, as this study was not designed to study the effects of the timing, frequency and intensity of burning and grazing scenarios on floristic diversity. The findings of Fynn, Morris & Edwards (2004) and others, as well as the patterns observed in the present study do, however, suggest that the effects of fire management on floristics within KZNSS requires further investigation.
Concluding remarks and recommendations
In terms of the future delimitations of KZNSS, the disturbances at IM indicate a possible shift in the floristic signature of improperly managed KZNSS sites: a reduction in species diversity and increased homogeneity relative to more properly managed and less disturbed sites (GGEP and SSNR). Studies on primary succession have revealed that disturbance in the short term leads to vegetation becoming less floristically distinct and, in the long term, leads to a unique, less diverse and more homogenous vegetation type (Walker & de Moral 2003). Despite the fact that IM contained the same number of diagnostic (endemic) taxa as the other sites, the use of endemic taxa as an indicator of site similarity is negated by the fact that all three sites contained only one of the 12 diagnostic taxa recognised for KZNSS. The absence of diagnostic species at all sites also indicated that past and present anthropogenic pressures associated with urbanisation might have altered the KZNSS floristic signature, making it less distinctive and more homogenous with other adjacent grassland types. However, this assertion will require extending the present study to other KZNSS patches and floristic comparisons between KZNSS patches and vegetation types such as KwaZulu-Natal Coastal Belt Grassland (CB3), which is floristically and environmentally similar to KZNSS (Mucina & Rutherford 2006).
Lastly, the results of the present study suggest that, given the geographic location of remnant patches of KZNSS (within urban and suburban matrix), the results of all subsequent floristic studies on KZNSS must be interpreted in relation to levels and types of management and disturbance. In this regard, given the highly transformed and limited extent of KZNSS nationally as well as provincially, the impact of management practices on floristics within KZNSS represents a research priority.
Acknowledgements
This research was supported by eThekwini Municipality through the Durban Research Action Partnership: KwaZulu-Natal Sandstone Sourveld Research Programme. The Wildlands Conservation Trust supported this research in part. Professor Ashley Nicholas and Mr Teddy Govender are thanked for their assistance in identification of difficult specimens. The officers in charge of the reserves are thanked for access: Thuthuka Majola of GGEP and Jabulani Khoza of SSNR. Numerous field assistants are thanked, especially Miss Saffron Mace for hours of fieldwork and data compilation assistance.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
C.D. was the main author, undertaking data collection, species identification, data compilation, data analyses and writing of the manuscript. S.R., S.N. and C.C. contributed to conceptualisation, realisation of field work, species identification, guidance in and interpretation of data analyses, writing and editing. R.B. and P.M. contributed to field work, species identification, data collection and compilation.
References
Bell, F.G. & Lindsay, P., 1999, 'The petrographic and geomechanical properties of some sandstones from the newspaper member of the Natal Group near Durban, South Africa' Engineering Geology 53, 57-81. http://dx.doi.org/10.1016/S0013-7952(98)00081-7 [ Links ]
Benson, D.H., 1985, 'Maturation periods for fire-sensitive shrub species in Hawkesbury Sandstone vegetation', Cunninghamia 1, 339-349. [ Links ]
Bond, W.J., Midgley, J. & Vlok, J., 1988, 'When is an island not an island? Insular effects and their cause in fynbos shrublands', Oecologia 77, 515-521. http://dx.doi.org/10.1007/BF00377267 [ Links ]
Brand, R.F., Brown, L.R. & du Preez, P.J., 2010, 'A floristic analysis of the vegetation of Platberg, eastern Free State, South Africa', Koedoe 52, Article #710. [ Links ]
Collins, S.L., Glenn, S.M. & Gibson, D.J., 1995, 'Experimental analysis of intermediate disturbance and initial floristic composition: Decoupling cause and effect', Ecology 76, 486-492. http://dx.doi.org/10.2307/1941207 [ Links ]
Colwell, R.K., 2013, 'EstimateS 9.1.0: Statistical estimation of species richness and shared species from samples', viewed 5 July 2014, from http://viceroy.eeb.uconn.edu/EstimateS/EstimateSPages/EstimateSRegistration.htm [ Links ]
Curtis, J.T. & Cottom G., 1956, 'Plant ecology work book', Laboratory field reference manual, Burgess Publ. Co., MN, pp. 39. [ Links ]
Department of Environmental Affairs, South African Government, 2011, 'National list of ecosystems that are threatened and in need of protection', Government Gazette 34809, 248-249. [ Links ]
Department of Environmental Affairs (DEA), South African Government, 2014, National environmental management: Biodiversity act (10/2004): Alien and invasive species list, Government Gazette 37886. [ Links ]
Fynn, R.W.S., Morris, C.D. & Edwards, T.J., 2004, 'Effect of burning and mowing on grass and forb diversity in a long-term grassland experiment', Applied Vegetation Science 7, 1-10. http://dx.doi.org/10.1111/j.1654-109X.2004.tb00589.x [ Links ]
Jonas, Z., Rouget, M., Reyers, B., Mohamed, B., Rutherford, M.C., Mucina, L. et al., 2006, 'Vulnerability assessment of vegetation types', in L. Mucina & M.C. Rutherford (eds.), The vegetation of South Africa, Lesotho and Swaziland, pp. 739-747, Strelitzia 19, SANBI, Pretoria, South Africa. [ Links ]
Kindt, R. & Coe, R., 2005, 'Tree diversity analysis', A manual and software for common statistical methods for ecological and biodiversity studies, World Agroforestry Centre (ICRAF), Nairobi. [ Links ]
Koleff, P., Gaston, K.J. & Lennon, J.J., 2003, 'Measuring beta diversity for presence-absence data', Journal of Animal Ecology 72, 367-382. http://dx.doi.org/10.1046/j.1365-2656.2003.00710.x [ Links ]
Luis, A.B., 1996, 'Chapter 6: Relationships between biotic diversity and primary productivity in savanna', in O.T. Solbrig, E. Medina & J.F. Silva (eds.), Biodiversity and savanna ecosystem processes, pp. 97-117, Springer, Berlin. [ Links ]
Maurin, O., Davies, T.J., Burrows, J.E., Daru, B.H., Yessoufou, K., Muasya, A.M. et al., 2014, 'Savanna fire and the origins of the 'underground forests' of Africa', New Phytologist, 204, 201-214. http://dx.doi.org/10.1111/nph.12936 [ Links ]
Mucina, L. & Rutherford, M.C., 2006, The vegetation of South Africa, Lesotho and Swaziland, Strelitzia 19, South African National Biodiversity Institute, Pretoria. [ Links ]
Mucina, L., Scott-Shaw, C.R., Rutherford, M.C., Camp, K.G.T., Matthews, W.S., Powrie, L.W. et al., 2006, 'Indian Ocean coastal belt', in L. Mucina & M.C. Rutherford (eds.), The vegetation of South Africa, Lesotho and Swaziland, pp. 569-583, Strelitzia 19, SANBI, Pretoria, South Africa. [ Links ]
Nagendra, H., 2002, 'Opposite trends in response for the Shannon and Simpson indices of landscape diversity', Applied Geography 22, 175-186. http://dx.doi.org/10.1016/S0143-6228(02)00002-4 [ Links ]
Parr, C.L., Lehmann, C.E.R., Bond, W.J., Hoffmann, W.A. & Andersen, A.N., 2014, 'Tropical grassy biomes: Misunderstood, neglected, and under threat', Trends in Ecology and Evolution 29, 205-213. http://dx.doi.org/10.1016/j.tree.2014.02.004 [ Links ]
Pinto, M.F., Nabinger, C., Boldrini, I.I., de Abreu Ferreira, P.M., Setubal, R.B., Trevisan, R., et al., 2013, 'Floristic and vegetation structure of a grassland plant community on shallow basalt in southern Brazil', Acta Botanica Brasillica 27, 162-179. http://dx.doi.org/10.1590/S0102-33062013000100017 [ Links ]
R Core Team, 2016, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, viewed 10 April 2015, from https://www.R-project.org/ [ Links ]
Rouget, M., Jonas, Z., Cowling, R.M., Desmet, P.G., Driver, A., Mohamed, B., et al., 2006, 'Ecosystem status and protection levels of vegetation types', in L. Mucina & M.C. Rutherford (eds.), The vegetation of South Africa, Lesotho and Swaziland, pp. 725-737, Strelitzia 19, SANBI, Pretoria, South Africa. [ Links ]
Rutherford, M.C., Mucina, L., Lotter, M.C., Bredenkamp, G.J., Smit, J.H.L., Scott-Shaw, R. et al., 2006, 'Savanna', in L. Mucina & M.C. Rutherford (eds.), The vegetation of South Africa, Lesotho and Swaziland, pp. 511-512, Strelitzia 19, SANBI, Pretoria, South Africa. [ Links ]
South African National Biodiversity Institute (SANBI), 2015, 'Red list of South African plants', viewed 10 April 2015, from https://redlist.sanbi.org [ Links ]
Walker, L. & del Moral, R., 2003, 'Chapter 7: Successional patterns', in L. Walker & R. del Moral (eds.), Primary succession and ecosystem rehabilitation, pp. 247, Cambridge University Press, New York. [ Links ]
 Correspondence:
Correspondence:
Syd Ramdhani
ramdhani@ukzn.ac.za
Received: 18 June 2016
Accepted: 10 Aug. 2016
Published: 03 Dec. 2016
Summary of species found in the present study. Alien and red list status of indigenous species are provided (according to the South African Biodiversity Institute Red List (SANBI, http://www.redlist.sanbi.org)) (abbreviations NE = not evaluated; LC = least concern; ; NT = near threatened; VU = vulnerable; EN = endangered), as well as herbarium details (Ward Herbarium; CCD = C.C. Drury) for specimens collected during the study (X denotes a species was found in a particular site; hyphen denotes absence).