Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 n.1 Pretoria 2024
https://doi.org/10.36303/jsava.587
ORIGINAL RESEARCH
Comparison of the immobilisation and cardiorespiratory effects of thiafentanil-azaperone versus thiafentanil-medetomidine-azaperone in African buffalo (Syncerus caffer)
VE FaberI; REJ BurroughsI, II; LCR MeyerII, III; HJ HansenIV; D GerberV; KN KoeppelI, II
IDepartment of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, South Africa
IICentre for Veterinary Wildlife Research, Faculty of Veterinary Science, University of Pretoria, South Africa
IIIDepartment of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, South Africa
IVWarmbad Dierekliniek, Bela-Bela, South Africa
VV-tech (Pty) Ltd, Midrand, South Africa
ABSTRACT
African buffalo (Syncerus caffer) are frequently immobilised for veterinary interventions, disease screening and translocations. Concerns over user and animal safety, costs, and irregularities in opioid supply, have led to the development of alternative immobilisation protocols. This study compared immobilisation of 12 boma-habituated African buffalo with thiafentanil-azaperone (TA) vs. thiafentanil-medetomidine-azaperone (TMA) in a randomised crossover study. Each buffalo received a combination of thiafentanil (6-7 mg) + azaperone (40 mg) and thiafentanil (1 mg) + medetomidine (3-4 mg) + azaperone (40 mg) with a three-week washout period between immobilisations. Induction and recovery times were recorded, quality of induction and immobilisation were scored subjectively, and physiological variables were monitored. The TMA combination induced immobilisation with 1/7th of the TA thiafentanil dose and at a quarter of the cost. Induction times for the TA combination were significantly faster at 5.7 ± 1.6 min and more reliable compared to the TMA combination at 10.95 ± 3.9 min. Both combinations resulted in severe hypoxaemia, however hypoxaemia was overall more pronounced in the TMA (PaO2 44 ± 14 mmHg) combination compared to the TA (PaO2 51 ± 13,33 mmHg) combination and resulted mainly from decreased pulmonary oxygen exchange rather than hypoventilation; PaCO2 values were mostly within the normal expected physiological range. Supplementary oxygen and close monitoring of blood oxygenation is considered essential with either combination. Although the reduction in costs could be beneficial for the wildlife industry, longer induction times, and risks from severe hypoxaemia need to be taken into consideration when the TMA combination is used.
Keywords: thiafentanil, medetomidine, azaperone, cardiorespiratory, African buffalo
Introduction
As a member of the "Big Five", the African buffalo (Syncerus caffer) is a sought-after animal for ecotourism and trophy hunting. Yet, buffalo have been associated with various diseases, namely foot-and-mouth disease (FMD), corridor disease, bovine brucellosis and bovine tuberculosis, which are of economic and public health importance. Thus, there is a greater need to immobilise them for disease screening, translocations, and veterinary interventions. Classically, African buffalo have been immobilised with potent opioids, etorphine and, or thiafentanil, in combination with a tranquiliser or sedative, or both (Kock & Burroughs 2021). Etorphine is a semi-synthetic derivate of the opioid alkaloid thebaine which is 1 000 x more potent than morphine (Blane et al. 1967; Zeiler & Meyer 2017). Thiafentanil, a fentanyl analogue, is a more recently developed synthetic opioid with exclusive μ-opioid receptor affinity (Vardanyan & Hruby 2014). It has a comparable potency to that of etorphine but a faster induction time and shorter duration of action (Lance & Kenny 2012; Zeiler & Meyer 2017). Based on the rapid and reliable immobilisation they provide, as well as their potent analgesic and sedative effects, etorphine and thiafentanil are commonly used as primary immobilisation agents in wild African herbivores (Grimm & Lamont 2007; Kock & Burroughs 2021). However, opioids are known to induce several side effects which may limit their clinical use. Commonly reported side effects are respiratory depression, CNS excitement, pulmonary hypertension, muscular hypertonicity and hyperthermia. Sub-optimal doses or severe agitation may result in prolonged excitement and sympathetic nervous system activation when opioids are used (Grimm & Lamont 2007; Kock & Burroughs 2021). To potentially mitigate these and other side effects, and to allow for reduced opioid dose requirements, opioids are often co-administered with sedatives or tranquilisers, or both. Of these drugs, the most commonly used are medetomidine, a highly selective a2-adrenergic receptor agonist, and azaperone, a butyrophenone drug. In combination, these drugs act synergistically with opioids; medetomidine and azaperone potentiate the immobilising effects of opioids thus allowing for reduced opioid dose requirements. Medetomidine induces deep sedation, analgesia and muscle relaxation in a dose-dependent manner by stimulating a2-adrenoreceptors at both pre- and post-synaptic levels. However, adverse effects such as vasoconstriction, resulting in increased systemic blood pressure and baroreceptor-mediated bradycardia, respiratory compromise, and thermoregulatory disturbances have also been reported (Kock & Burroughs 2021). Azaperone is a neuroleptic tranquilising drug with its main effects mediated via dopaminergic (D2) antagonism (Gaudio et al. 2020; Kock & Burroughs 2021). Since the 1980s, azaperone has been used to tranquilise wildlife species and as an adjunct with immobilising drugs to potentiate immobilisation (Grimm & Lamont 2007; Kock & Burroughs 2021). The addition of azaperone to immobilisation protocols provides a smoother induction and can also alleviate hypertension, particularly in rhino and elephant immobilisation (Kock & Burroughs 2021). At therapeutic doses, azaperone antagonises (a1-adrenoceptors resulting in peripheral vasodilation. These effects are considered beneficial in wildlife immobilisation as they counteract opioid- and (2-agonist-induced hypertension, as well as hypertension potentially induced by capture associated stress responses (Mentaberre et al. 2010). However, doses exceeding therapeutic recommendations can induce adverse effects such as extrapyramidal effects and catalepsy (Kock & Burroughs 2021).
Free ranging African buffalo have routinely been immobilised with thiafentanil and azaperone (TA) (Curro 2007; Kock & Burroughs 2021; Szabó et al. 2015). Alternative and non-potent opioid immobilising combinations have gained popularity over the last few years due to concerns over user safety, costs, and irregularities in opioid supply. An example of such a combination that is now being used in buffalo, and many other ruminant and non-ruminant species in southern Africa, is based on a relatively low thiafentanil dose in combination with medetomidine and azaperone (TMA). The opioid doses that are used in this combination are 20-30% of the routinely used standard species-specific doses and is a more affordable combination. As there is little published information available on the efficacy and safety of this alternative combination, the objective of this study was to compare the immobilisation and physiological effects, in particular the respiratory and cardiovascular effects, of the thiafentanil-azaperone combination (Curro 2007; Kock & Burroughs 2021; Szabó et al. 2015), and this low opioid dose thiafentanil-medetomidine-azaperone (Kock & Burroughs 2021) combination, in African buffalo.
Materials and methods
Prior to the commencement of the study, ethical approval was obtained from the Animal Ethics Committee of the University of Pretoria (REC 241-19).
Twelve boma-habituated African buffalo (Syncerus caffer) bulls between two-and-a-half to four years of age were used for this prospective, randomised crossover study which took place in October/November 2020 at Lekkerleef Wild Stoet located in the Sekhukhune District Municipality, Limpopo, South Africa (S:24°57"50.6', E 29°11"12.1'). All study animals were permanently housed in breeding camps. Immobilisation and data collection were carried out under a roofed boma of 30 x 50 m. The study was conducted over two data collection periods. Drug doses for the drug protocols used per individual were determined according to the estimated body mass and condition score (BCS) of each buffalo as observed by the team at the time of immobilisation.
During the first data collection period, six of the 12 buffalo were randomly assigned to be immobilised with the TA combination, and the remaining six buffalo with the TMA combination. After a three-week washout period, the buffalo were crossed over and each buffalo received the alternative combination. The doses of the drugs used in the combinations were calculated for a 450550 kg buffalo, as follows:
Thiafentanil-azaperone (TA): With the TA combination, each buffalo received 6-7 mg thiafentanil (Thianil®, 10 mg/ml, Wildlife Pharmaceuticals, White River, Mpumalanga, South Africa) and 40 mg azaperone (Azaperone, 100 mg/ml, V-tech (Pty) Ltd, Johannesburg, South Africa) intramuscularly.
Thiafentanil-medetomidine-azaperone (TMA): The TMA immobilisation combination contained a relatively low dose of 1 mg of thiafentanil with 3-4 mg medetomidine (Medetomidine HCl, 50 mg/ml, V-tech (Pty) Ltd, Johannesburg, South Africa) and 40 mg azaperone per buffalo intramuscularly.
The drugs were administered into the left or right gluteal muscle using a 1.5 cc Motsumi Stinger dart (Motsumi Darts®, Pretoria, South Africa) with a 1¼ inch wire-barbed needle projected over a 3 m-5 m distance from a Pneu-dart cartridge-fired dart projector (Model 389, Pneu-dart®, Williamsport, Pennsylvania, USA). Only one buffalo was immobilised and worked with at a time. Before dart placement, the respective buffalo was moved into a separate compartment. After the drugs were added to the dart, the dart chamber was filled up with physiological saline (Sodium Chloride Fresenius 0.9%, 1 000 ml, Fresenius Kabi Manufacturing SA (Pty) Ltd, Port Elizabeth, South Africa) to ensure that each buffalo received the same volume. Once the drugs were administered, the induction times were recorded. The time to first signs was defined as the time interval between dart placement and signs of ataxia, and the time to recumbency as the time between dart placement and when the buffalo became recumbent and could be handled safely. Quality of induction was evaluated subjectively using a qualitative 1-4 scale scoring system; excellent 1), good 2), fair 3), poor 4) (Laubscher et al. 2022).
Immobilisation monitoring
Once immobilised, the animal was placed into and maintained in sternal recumbency and instrumented within five minutes. Blindfolds were used to reduce exposure to external stimuli. The left or right medial auricular artery was aseptically prepared and cannulated with a 22-gauge catheter (Jelco®, Smiths Medical, Lancashire, United Kingdom) to allow for continuous arterial blood pressure measurements and blood sampling. The systolic (SAP), mean (MAP), and diastolic (DAP) arterial pressure were measured in 5-minute intervals using an intra-arterial pressure monitor (IntraTorr, IntraVitals, United Kingdom) which was connected to a pressure transducer with non-compliant tubing (Deltran Disposable Pressure Transducer DPT 200, Utah Medical Products Incorporated, USA). The pressure transducer was zero-calibrated at the level of the heart.
Before each sample collection, 0.5-1 ml waste blood was drawn and discarded. Blood samples were then collected into sodium-heparinised 1 ml syringes at 10, 20 and 35 minutes after reaching recumbency. The sample was analysed immediately with a portable blood gas analyser (epoc® SIEMENS Blood Analysis System, Siemens Healthcare GmbH, Erlangen, Germany) using single-use self-calibrating epoc® BGEM Test Cards. The blood values measured, at a stat temperature of 37 °C, were the partial pressure of carbon dioxide (PaCO2) and oxygen (PaO2), pH, blood lactate and glucose.
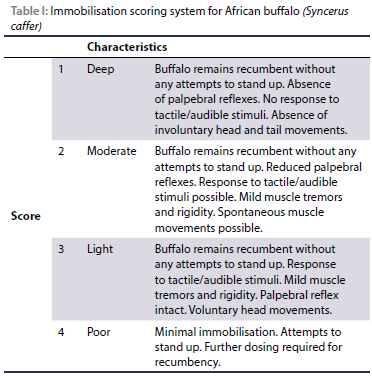
Heart rate (HR), respiratory rate (RR), body temperature (BT), peripheral oxygen haemoglobin saturation (SpO2), and end-tidal carbon dioxide (EtCO2) were recorded every five minutes for 40 minutes with the first reading being taken at five minutes post recumbency. The HR was determined by auscultation over 60 seconds, and respiratory rate by counting thoracic/abdominal excursions or air movement at the nares for 60 seconds. Peripheral oxygen haemoglobin saturation (SpO2) was measured using a pulse oximeter (Veterinary Pulse Oximeter, Model 9847V, Nonin Medical, USA) with the transflectance probe placed on the 3rd eyelid. A multi-parameter monitor (M3T Mini vet, TooToo Meditech, China) was used to measure end-tidal carbon dioxide (EtCO2) with a 100 mm internal diameter cuffed endotracheal tube placed into the nasal cavity. A digital thermometer (HI98509 Checktemp 1, HANNA Instruments (Pty) Ltd, USA) was inserted 100 mm into the rectum to measure body temperature (C°). The same device was used for the environmental temperature before each immobilisation. The quality of immobilisation and anaesthetic plane was recorded every five minutes using a 1-4 scoring system and was determined subjectively by the primary investigator: deep 1), moderate 2), light 3), poor 4) (Table I). At the end of first data collection period, after the last data collection point, each buffalo was placed on a scale. The scale consisted of a steal plate which was placed onto two load bars and which was connected to a digital readout instrument (Gallagher TW-1 Livestock Scale Indicator). The obtained true body weights of the buffalo were used to calculate the exact drug dose per kilogram body weight.
Recovery
After the last data collection point at 40 minutes postinstrumentation, each buffalo was de-instrumented, and the immobilisation drugs antagonised. The TA combination was antagonised with naltrexone (Naltrexone HCl, 40 mg/ ml, Kyron Laboratories (Pty) Ltd, Johannesburg, South Africa) intravenously in the caudal auricular vein. For each mg of thiafentanil, 10 mg of naltrexone was administered. The TMA combination was antagonised with a standardised mixture of yohimbine (Yohimbine, 6.25 mg/ml, Kyron Laboratories (Pty) Ltd, Johannesburg, South Africa) and atipamezole (Atipamezole, 20 mg/ml, V-tech, Johannesburg, South Africa) intravenously. The atipamezole-yohimbine combination contained 2mg of atipamezole for each ml of yohimbine. Each animal received 0.5 ml atipamezole-yohimbine mixture per mg medetomidine, which equates to 1 mg atipamezole and 3.125 mg of yohimbine per mg medetomidine. Naltrexone was given at the previously mentioned dose into the same caudal auricular vein.
Data analysis
Data analysis was performed using commercially available IBM SPSS Statistical software (IBM SPSS Statistics Version 25.0; International Business Machines Corp; Released 2017; NY, USA) and evaluated for normality by calculation of descriptive statistics, plotting histograms, and by performing a Shapiro-Wilk test. Quantitative data were described using mean, ± standard deviation (SD) and ranges. Results were interpreted at a 5% significance level (p < 0.05). Paired sample t-tests were used to compare the clinical and physiological data obtained between the two combinations, at each time point, over 40 minutes.
The alveolar oxygen tension (PAO2) was calculated from the alveolar gas equation PAO2 = (Patm - PH2O) FiO2 - PaCO2/RQ. The A-a gradient is the difference between the alveolar oxygen partial pressure (PAO2) and the arterial oxygen partial pressure (PaO2): A-a Gradient = PAO2 - PaO2 (Sarkar et al. 2017). FiO2 is the fractional concentration of inspired oxygen (21%), Patm is the atmospheric pressure calculated at an altitude of 910 m for the Marble Hall district (683 mmHg), PH2O the water vapour pressure of saturated air in the alveoli (47 mmHg) and RQ (=1) the gas exchange ratio (Shah et al. 2014). Total costs of drugs associated with the chemical immobilisation of the buffalo with the TA and TMA regimen were calculated and compared descriptively.
Results
The age of the buffalo ranged between two-and-a-half and four years and the mean body mass was 511 kg (Range: 428.5-635 kg) at the time of the study. The mean actual dose of the thiafentanil-azaperone combination was 0.0136 mg/kg thiafentanil (Range: 0.011-0.0163 mg/kg) + 0.0792 mg/kg azaperone (Range: 0.063-0.093 mg/kg); and 0.00216 mg/kg thiafentanil (Range: 0.0016-0.0023 mg/kg) + 0.00688 mg/kg medetomidine (Range: 0.0047-0.0084 mg/kg) + 0.0688 mg/kg azaperone (Range: 0.0470.084 mg/kg) for the thiafentanil-medetomidine-azaperone combination.
Two of the 12 buffalo had to be excluded from the study; one due to an inability to place an arterial catheter and the other one due to an inadequate dart placement. In total, 10 out of 12 buffalo were included in the analysis of the data. Of these, all were successfully immobilised for a minimum of 40 minutes regardless of which protocol had been used. Two of these buffalo, both immobilised with the TMA combination, did not reach immobilisation on their own within a timeframe of 20 minutes post-dart placement. However, these buffalo could be approached safely and could be assisted into recumbency. None of the buffalo included in the data analysis required any additional drugs to reach immobilisation or to remain recumbent for the duration of the procedures.
The mean time from darting to recumbency for TA was 5.7 ± 1.58 minutes (Range: 4-9.5 minutes) which was significantly (p < 0.001) different from 10.95 ± 3.92 minutes (Range: 6-20 minutes) for the TMA combination. Induction quality tended to be superior in the TA compared to the TMA combination as represented by the higher induction score for the TMA protocol (Table II).
There was no significant difference between the immobilisation quality score for the two combinations. The mean immobilisation score was 1 and 1.4 for the TMA and TA combination, respectively. However, more buffalo immobilised with the TA combination showed an immobilisation score of greater than 1 (n = 5) compared to the TMA combination (n = 2). One animal immobilised with the TA combination showed a light and insufficient plane of immobilisation towards the end of the procedure (at T = 35). Visual observation revealed differences in characteristics of immobilisation between the two immobilisation protocols. Buffalo immobilised with the TA combination showed generalised muscular rigidity and noticeable muscle tremors and involuntary movements predominantly in the ears, head and tail area despite being fully immobilised. One animal immobilised with the TA combination showed signs of regurgitation. Immobilisation with the TMA combination was characterised by a more regular breathing pattern, as well as the absence of involuntary movements and muscle rigidity.
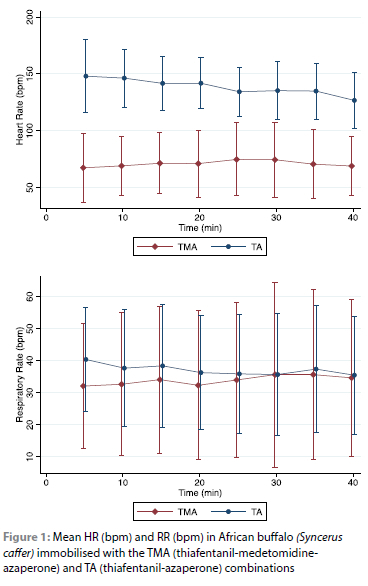
The HR was significantly different (p < 0.001) between the two immobilisation protocols and was on average higher in the TA combination. A mean HR of 139 ± 25 bpm (Range: 100-179 bpm) was measured in the TA combination with a transient, non-significant decrease over time. A mean HR of 70 ± 27 bpm (Range: 51-144 bpm) was recorded in the TMA combination with stable values over time (Figure 1).
The immobilisations took place in a wide range of environmental ambient temperatures (24.5-37.4 °C). No significant differences (p < 0.217) were found when comparing the BT between the two immobilisation protocols. A mean body temperature of 40.5 ± 1.3 °C (Range: 38.8-43.8 °C) for the TMA and 40.3 ± 0.8 °C (Range: 39.2-41.8 °C) for the TA combination was recorded. All buffalo developed hyperthermia (> 39.5 °C). No statistically significant linear relationship was found between environmental and BT.
The RR was similar between the two combinations (p < 0.220). The mean breaths per minute were 33 ± 22 (Range: 13-83) and 37 ± 18 (Range: 9-64) for TMA and TA, respectively (Figure 1). Respiratory rhythm in the TMA combination appeared subjectively more regular with even chest expansions (inspiratory depth) compared to the TA combination.
The SpO2 was significantly different between the two combinations (p < 0.024). All buffalo were considered hypoxaemic. However, buffalo were significantly less hypoxaemic [SpO2: 73 ± 12% (Range: 54-88%)] when the TA combination was used compared to the TMA combination [SpO2: 67 ± 17% (Range: 48-97%)]. In the TMA combination, SpO2 decreased significantly (p < 0.037) between T5 and T20 from 73% to 64%.
No significant difference (p < 0.068) was found when comparing the EtCO2 results.
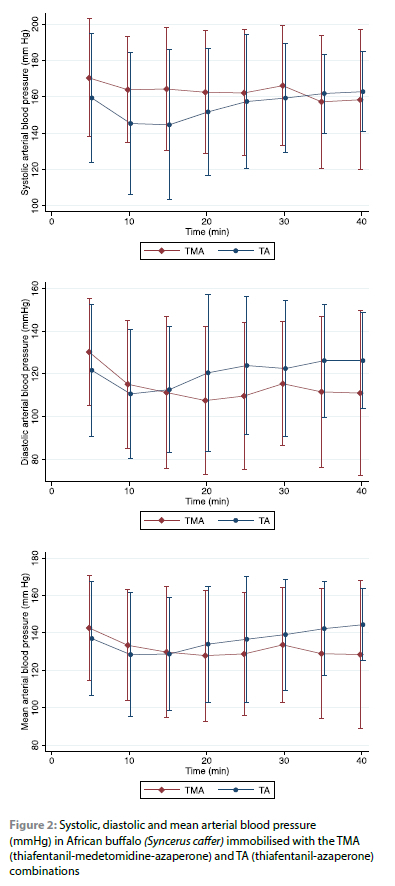
There was no significant difference in the systolic (p < 0.157), diastolic (p < 0.108) and mean (p < 0.266) blood pressure between the two combinations (Figure 2).
There was a significant difference in the PaO2 (p < 0.05) between the two combinations. The PaO2 was significantly lower in the TMA combination [PaO2: 44 ± 14 mmHg (Range: 24-77 mmHg)] compared to the TA combination [PaO2: 51 ± 13 mmHg (Range: 33-80 mmHg)] (p = 0.032) (Figure 3).
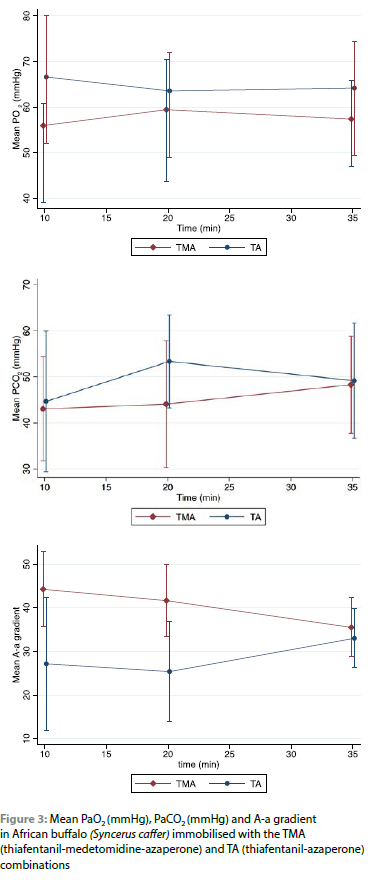
There was no significant difference (p < 0.096) between the PaCO2 values between the two combinations and values appeared stable over time. The mean PaCO2 level was 44 ± 10 mmHg in the TA and 39 ± 11 mmHg in the TMA combination (Figure 3).
The A-a gradient was significantly different between the two combinations (p < 0.001) with a mean of 27 ± 13 mmHg (Range: 8-41 mmHg) in buffalo immobilised with the TA, and 40 ± 9 mmHg (Range: 33-57 mmHg) with the TMA combination (Figure 3).
A significant difference (p < 0.001) was found in the blood pH between the two treatment groups, the mean pH value was 7.39 ± 0.54 (Range: 7.33-7.48) and 7.33 ± 0.52 (Range: 7.3-7.42) in the TMA and TA combination, respectively.
There was no significant difference between the two combinations with regards to the glucose (p < 0.072) and lactate (p < 0.505) levels. The mean glucose level was 9.24 ± 2.91 mmol/l (Range: 5.8-14.5 mmol/l) and 7.96 ± 2.49 mmol/l (Range: 5.213.8 mmol/l) in the TMA and TA combination, respectively. Mean lactate concentration was 4.76 ± 4.19 mmol/l (Range: 1.15-12.82 mmol/l) in the TA and 3.99 ± 3.82 mmol/l (Range: 0.73-11.86 mmol/l) in the TMA combination.
There was a significant difference (p < 0.022) between the two combinations with regards to the recovery times. The mean time to recovery was 80 ± 33 seconds (Range: 30-130 seconds) and 95 ± 30 seconds (Range: 50-140 seconds) in the TA and TMA combination, respectively. No difference in the quality of recovery was observed between the two combinations.
All buffalo were monitored for 24 hours post immobilisation. No signs of re-narcotisation were observed.
Immobilisation with the TMA combination was at a quarter of the cost of the TA combination.
Discussion
Both combinations were effective in providing a reliable immobilisation for a minimum of 40 minutes with quick recoveries and no mortalities. Thus, either combination would be suitable for routine veterinary and management procedures in boma-habituated buffalos. However, there are important clinical findings and differences between the two combinations, in particular induction times and certain physiological variables, which need to be highlighted and considered.
Immobilisation with the TA combination resulted in significantly shorter induction times with superior induction scores compared to the TMA combination. Induction with the TA combination was characterised by a high-stepping gait and hypermetria before reaching sternal recumbency. Immobilisation with the TMA combination resulted in a more variable induction: some buffalo showed a relatively smooth induction with a wide-based stance and reduced physical activity; others showed visible excitement after drug administration with marked pacing. The larger SD and range in induction times in the TMA group indicates that immobilisation with this combination resulted in a less reliable induction, possibly due to different individual responses to the capture protocol and perceived stressors. Non-domestic and domestic bovids are sensitive to capture stress. The sedative effects of medetomidine are dose-dependent. However, severe excitation, stress and anxiety can result in the release of catecholamines which interfere with the a2-agonists and consequently delay or prevent the onset of sedation. In that case, higher doses are required to achieve sufficient sedation or immobilisation (Caulkett & Haigh 2007; De Lange et al. 2017). A greater stress response in some buffalos could have delayed or prevented the onset of sedation in the TMA treatment resulting in slower inductions in these animals. Given the significant increase in induction times in the TMA treatment, this combination, at these selected doses, should preferably be reserved for immobilisations in bomas or small camps. In contrast, induction with the TA combination was more consistent, providing a more predictable induction. Under extensive field conditions, reliability, quality and length of induction are of clinical relevance. Shorter induction times can mean less distance travelled by the animal which can minimise the risk of capture- and stress-related morbidities and mortalities, and further ensure that the animal can be found and handled as early as possible.
The heart rate showed a significant difference (p < 0.01) between the two combinations. Due to the lack of scientific papers on the physiological variables in African buffalo, the average heart rate was calculated allometrically. An average heart rate of 57 bpm (Range: 53-59 bpm) in buffalo was estimated according to Mortola and Lanthier (2004) with proposed formula: HR = 320 x BW-0.28. Compared to the calculated physiological heart rate, none of the animals of the TMA or the TA combination was found to be bradycardic. The significant lower heart rate observed in buffalo immobilised with the TMA combination could have resulted from both central and peripheral a2-adrenergic receptor activation. Alpha2-adrenergic agonists can induce dose-dependent cardiovascular side-effects such as bradycardia with associated bradyarrhythmia, 2nd-degree arterio-ventricular blocks, reduced cardiac output and transient hypertension followed by hypotension (Sinclair 2003). Opioids can induce both tachycardia and bradycardia depending on the species and type of opioid used (Zeiler & Meyer 2017). In this study, most animals immobilised with the TA combination were found to be tachycardic. The increase in heart rate can be caused by direct opioid-induced sympathetic nervous system activation and catecholamine release or indirectly as a response to hypoxaemia and hypercapnia (Grace et al. 2021).
Ruminants are sensitive to anaesthesia-induced effects on the respiratory system. Hypercapnia and hypoxaemia are the most common side-effects of anaesthesia or deep sedation in ruminants (Seddighi & Doherty 2016). As minute ventilation was not directly measured in this study, quality of ventilation was determined by the respiratory rate and measured PaCO2. If ventilation is impaired, PaCO2 increases. According to the recorded PaCO2 values, 11 out of 20 buffalo - five of the TMA combination and six of the TA combination - were considered hypercapnic (PaO2 > 42 mmHg). However, PaCO2 values were not severely elevated in any buffalo, suggesting adequate ventilation in both combinations. Clinically relevant hypoxaemia was observed with both combinations, although more severe in the TMA than the TA combination (p = 0.032). According to the recorded PaO2 values, seven out of 20 buffalo - five of the TMA combination and two of the TA combination, were considered as severly hypoxaemic (< 40 mmHg). The presence of hypoxaemia was further mirrored by the widened alveolar-arterial gradient (A-a gradient), especially in the TMA combination. The alveolar-arterial gradient (A-a gradient) measures the difference of alveolar oxygen concentration and arterial oxygen concentration and is used to help identify the source of hypoxaemia. In animals, an A-a gradient above 25 mmHg is considered abnormal (Grimm 2008) and indicates that hypoxaemia was likely caused by impaired alveolar gas exchange resulting in oxygen diffusion deficits. Due to the widened A-a gradient in the face of mostly normocapnia in the buffalo, and a respiratory rate being within and above the normal physiological range (18-30 bpm) (Grace et al. 2021), the origin of hypoxaemia was more likely due to ventilation/ perfusion mismatch (V/Q mismatch), oxygen diffusion impairment and/or shunting rather than hypoventilation (Celly et al. 1997; Hantzidiamantis & Amaro 2020). Although not considered too relevant in this study since the buffalo were in sternal (ventral) recumbency, ventilation-perfusion mismatch (V/Q ratio) can result from abnormal positioning of the animal during immobilisation causing lung compression and changes in tidal volume due to an increased weight of viscera on the diaphragm (Read 2003; Seddighi & Doherty 2016). Therefore, it was speculated that direct drug-induced changes were the primary reason for the observed hypoxaemia in all the buffalo. The pulmonary effects of a2-agonist administration have not been studied in buffalo, but previous studies in ruminants have shown that administration of a2-adrenoreceptor agonists can trigger the activation of pulmonary intravascular macrophages (PIM) resulting in the release of vasoactive substances and the development of pulmonary hypertension and an increased transit time of blood flow through the pulmonary capillary beds resulting in oxygen diffusion impairment (Kästner 2006; Seddighi & Doherty 2016). Furthermore, medetomidine can cause peripheral vasoconstriction mostly by direct activation of endothelial a2-adrenoreceptors delaying the blood flow through peripheral tissues and resulting in increased oxygen consumption (Käster 2006; Zeiler & Meyer 2017). In small ruminants, peripheral vasoconstriciton and increased transpulmonary pressure following a2-agonist administration can result in the development of pulmonary congestion and oedema which may hinder gas exchange and result in hypoxaemia (Celly et al. 1997; Seddighi & Doherty 2016). It is speculated that the hypoxaemia observed in the TMA treatment was primarily mediated, or at least part thereof, by changes in the airway mechanics and haemodynamics associated with increased pulmonary pressures, V/Q mismatching, shunting and dead space ventilation resulting in oxygen diffusion impairment as mirrored by the widened A-a gradient. Similar effects were believed to occur in hypoxaemic blesbok which were immobilised with a similar TMA combination (Roug et al. 2023).
Opioid-induced respiratory depression is a well described side-effect following the administration of etorphine and thiafentanil and is mainly mediated by reducing the ventilatory response to hypoxaemia and hypercapnia. In addition, opioids can negatively influence respiratory rhythmogenesis, as well as the chest wall expansion in a dose-dependent manner (Buss et al. 2015). The widened A-a gradient in the buffalo suggests that oxygen diffusion impairment, ventilation/perfusion mismatch and right-to-left shunting may have contributed to the hypoxaemia seen. In previous studies, etorphine administration in goats resulted in a widened A-a gradient due to the development of pulmonary hypertension and possible oedema resulting in impaired gas diffusion (Meyer et al. 2015). Pulmonary hypertension can result from an increased pulmonary vascular resistance caused by both direct (drug) and indirect (hypoxia) sympathetic system activation as well as other opioid-induced effects on the pulmonary vasculature (Meyer et al. 2015). Pulmonary hypertension may impair oxygen diffusion as rapid blood flow across the pulmonary capillary beds shortens the blood transit time and therefore the time for gaseous exchange (Meyer et al. 2015). Another factor that could have contributed to the observed widened A-a gradients and hypoxaemia in the TA combination is an increase in oxygen consumption. Sympathetic system activation following exogenous opioid administration can increase cellular metabolism and oxygen consumption (Buss et al. 2018). The overall increased muscle rigidity observed in the TA group is a frequently reported observation of opioids and is believed to be mediated through μ-opioid receptor activation (Zeiler & Meyer 2017). Opioid-induced muscle rigidity and involuntary tremors can be concerning as increased tissue metabolism can result in oxygen depletion, lactic acidosis and associated consequences (De Lange et al. 2017). Although the medetomidine in the TMA combination appeared to reduce muscle rigidity and likely contributed to the improved immobilisation quality, it had no apparent effect on preventing acidosis and appeared to worsen the hypoxaemia observed.
Based on the measured respiratory rates, and PaCO2 values being mostly within the normal physiological range, it is important to emphasise that despite adequate ventilation in the buffalo, hypoxaemia was significantly more profound in the TMA combination. Supplementary oxygen should be considered essential during immobilisation with either combination, but its efficacy should be continuously assessed, especially when the TMA combination is used considering that shunting may be profound with this combination. In future studies, interventions should not only be focused on improving oxygen delivery to the alveoli but also to improve the oxygen exchange between the alveoli and arterial blood, possibly by preventing pulmonary vasoconstriction and hypertension (Meyer et al. 2015).
The TA combination may benefit from the addition of the mixed opioid agonist-antagonist butorphanol, administered post recumbency as this could counteract the undesired μ-opioid receptor effects such as pulmonary hypertension (and resulting oxygen exchange deficits), as well as muscular hypertonicity and tremors, while maintaining the sedative and analgesic κ-opioid receptor-mediated effects (Bush et al. 2001). When using the TMA combination, the addition of vatinoxan (MK-467), a peripheral a2-receptor antagonist, may also help improve safety during the immobilisation. The latter could improve cardiovascular function by alleviating hypertension and bradycardia brought about by a2-receptor agonists (Einwaller et al. 2020).
The large standard deviation seen, particularly in induction times, could indicate different responses of specific individuals to certain drug combinations or to perceived stressors. To compensate for errors of measurement, or to better quantify individual responses, a larger sample size may have been of value. As this was not a blinded study, qualitative values could have been biased. This study took place in a controlled boma environment where the drug effects in buffalo could differ compared to other environments, like free-ranging conditions.
Conclusion
The TA combination induced immobilisation in a more predictable manner and in times that were significantly quicker to those induced by TMA. A clinically relevant hypoxaemia was present with both combinations, although more severe in the TMA than the TA combination, and is thought to be mediated primarily, or at least part thereof, by changes in the airway mechanics and haemodynamics resulting in oxygen diffusion impairment rather than hypoventilation. When using these combinations, oxygen supplementation should be provided, and its efficacy continuously assessed. Future interventions aimed at improving pulmonary oxygen exchange in immobilised buffalo should be investigated. Given the significant increase in induction times and the greater level of hypoxaemia in the TMA combination, this combination, at the selected doses, should be reserved for controlled environments where stressors can be kept to a minimum.
Acknowledgements
The authors would like to thank the University of Pretoria, Warmbad Dierekliniek and V-Tech (Pty) Ltd Pharmaceutical for the financial support. We thank Frans Stapelberg and his team at Lekkerlief Wild Stoet for providing their facilities and animals for this study.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The study was approved by the Animal Ethics Committees of the University of Pretoria (REC 241-19). All institutional and national guidelines for the care and use of animals were followed.
ORCID
VE Faber https://orcid.org/0009-0005-3946-6363
REJ Burroughs https://orcid.org/0000-0003-0962-8120
LCR Meyer https://orcid.org/0000-0002-5122-2469
HJ Hansen https://orcid.org/0000-0001-5860-8981
KN Koeppel https://orcid.org/0000-0001-5816-9245
References
Blane, G.F., Boura, A.L.A., Fitzgerald, A.E., et al., 1967, Actions of etorphine hydrochloride, (M99): A potent morphine-like agent, British Journal of Pharmacology and Chemotherapy 30, 1 1-22. https://doi.org/10.1111/j.1476-5381.1967.tb02108.x. [ Links ]
Bush, M., Citino, S., Lance, W., 2001, The use of butorphanol in anaesthesia protocols for zoo and wild mammals, in E. Miller & M. Fowler (eds.), Fowler's Zoo and Wild Animal Medicine, pp. 596-603, Saunders, Philadelphia. https://doi.org/10.1016/B978-1-4377-1986-4.00077-9. [ Links ]
Buss, P., Olea-Popelka, F., Meyer, L., et al., 2015, Evaluation of cardiorespiratory, blood gas, and lactate values during extended immobilization of white rhinoceros (Ceratotherium simum), Journal of Zoo and Wildlife Medicine 46(2), 224-233. https://doi.org/10.1638/2014-0089R.1. [ Links ]
Buss, P., Miller, M., Fuller, A., et al., 2018, Post induction butorphanol administration alters oxygen consumption to improve blood gases in etorphine-immobilised white rhinoceros, Veterinary Anaesthesia and Analgesia 45(1), 57-67. https://doi.org/10.1016/j.vaa.2017.03.008. [ Links ]
Caulkett, N., Haigh, J.C., 2007, Bison, in G. West, D. Heard & N. Caulkett (eds.), Zoo Animal and Wildlife Immobilisation and Anaesthesia, pp. 643-646, John Wiley and Sons, Ames. https://doi.org/10.1002/9780470376478.ch58. [ Links ]
Celly, C.S., McDonell, W.N., Young, S.S., et al., 1997, The comparative hypoxemic effect of four a2 adrenoceptor agonists (xylazine, romifidine, detomidine and medetomidine) in sheep, Journal of Veterinary Pharmacology and Therapeutics 20, 464-471. https://doi.org/10.1046/j.1365-2885.1997.00097x [ Links ]
Curro, T.G., 2007, Non-domestic cattle, in G. West, D. Heard & N. Caulkett (eds.), Zoo Animal and Wildlife Immobilisation and Anaesthesia, pp. 635-642, John Wiley and Sons, Ames. https://doi.org/10.1002/9780470376478.ch57. [ Links ]
De Lange, S., Fuller, A., Haw, A., et al., 2017, Tremors in white rhinoceroses (Ceratotherium simum) during etorphine-azaperone immobilisation, Journal of the South African Veterinary Association 88(0), 1466. https://doi.org/10.4102/jsava.v88i0.1466. [ Links ]
Einwaller, J., Painer, J., Raekallio, M., et al., 2020, Cardiovascular effects of intravenous vatinoxan (MK-467) in medetomidine-tiletamine-zolazepam anaesthetized red deer (Cervus elaphus), Veterinary Anaesthesia and Analgesia 47(4), 518-527. https://doi.org/10.1016/j.vaa.2019.10.011. [ Links ]
Gaudio, E., Laubscher, L.L., Pfitzer, S., et al., 2020, Immobilization quality and cardiopulmonary effects of etorphine alone compared with etorphine-azaperone in blesbok (Damaliscus pygargus phillipsi), Veterinary Anaesthesia and Analgesia 47(4), 528-536. https://doi.org/10.1016/j.vaa.2019.10.012. [ Links ]
Grace, J.F., Miller, M.A., Raath, J.P., Laubscher, L.L., Buss, P.E., Zeiler, G.E., 2021, Immobilization of African buffalo (Syncerus caffer) using etorphine-midazolam compared with etorphine-azaperone, Veterinary Anaesthesia and Analgesia 48 (5), 734-744. https://doi.org/10.1016/j.vaa.2021.03.018. [ Links ]
Grimm, K.A., Lamont, L.A., 2007, Clinical Pharmacology, in G. West, D. Heard & N. Caulkett (eds.), Zoo Animal and Wildlife Immobilisation and Anaesthesia, pp. 3-36, John Wiley and Sons, Ames. [ Links ]
Grimm, K.A., 2008, Arterial blood gas analysis and interpretation in small animal practice (Proceedings). https://www.dvm360.com/view/arterial-blood-gas-analysis-and-interpretation-small-animal-practice-proceedings. [ Links ]
Hantzidiamantis, P.J., Amaro, E., 2020, Physiology, Alveolar to Arterial Oxygen Gradient, StatPearls Publishing, Treasure Islands, Florida. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545153/. [ Links ]
Kästner, S.B.R, 2006, A2-agonists in sheep: A review, Veterinary Anaesthesia and Analgesia 33, 79-69. https://doi.org/10.1111/j.1467-2995.2005.00243x [ Links ]
Kock, M.D., Burroughs, R.E., 2021, Chemical and Physical Restraint of African Wild Animals, Third Edition, Michael D. Kock, International Wildlife Veterinary Services (Africa), Greyton, South Africa. [ Links ]
Lance, W.R., Kenny, D.E., 2012, Thiafentanil oxalate (A3080) in nondomestic ungulate species, in R.E. Miller & M.E. Fowler (eds.), Fowler's Zoo and Wild Animal Medicine Current Therapy, pp. 589-595, Elsevier Health Sciences, New York. https://doi.org/10.1016/B978-1-4377-1986-4.00076-7. [ Links ]
Laubscher, L.L., Meyer, L.C.R., Laurence, M., et al., 2022, A comparison of immobilisation quality and cardiorespiratory effects of etorphine-azaperone versus etorphine-midazolam combinations in blesbok, Journal of the South African Veterinary Association 93(1), 1-9. https://doi.org/10.36303/JSAVA.2022.93.1.491. [ Links ]
Meyer, L.C.R., Hetem, R.S., Mitchell, D., et al., 2015, Hypoxia following etorphine administration in goats (Capra hircus) results more from pulmonary hypertension than from hypoventilation, BMC Veterinary Research 11(18), 1-9. https://doi.org/10.1186/s12917-015-0337-5. [ Links ]
Mentaberre, G., López-Olvera, J.R., Casas-Díaz, E., et al., 2010, Effects of azaperone and haloperidol on the stress response of drive-net captured Iberian ibexes, European Journal of Wildlife Research 56 (5), 757-764. https://doi.org/10.1007/s10344-010-0371-3. [ Links ]
Mortola, J.P., Lanthier, C., 2004, Scaling the amplitudes of the circadian pattern of resting oxygen consumption, body temperature and heart rate in mammals, Comparative biochemistry and physiology. Part A, Molecular & Integrative Physiology 139 (1), 83-95. https://doi.org/10.1016/j.cbpb.2004.07.007. [ Links ]
Read, M.R., 2003, A review of alpha2-adrenoreceptor agonists and the development of hypoxaemia in domestic and wild ruminants, Journal of Zoo and Wildlife Medicine 34(2), 134-138. https://doi.org/10.1638/1042-7260(2003)034[0134:AROAAA]2.0.CO;2. [ Links ]
Roug, A., Smith, C., Raath, J.P., et al., 2023, Efficacy and safety of three different opioid-based immobilisation combinations in blesbok (Damaliscus pygargus phillipsi), Journal of South African Veterinary Association 94, 145-152. https://doi.org/10.4102/jsava.v88i0.1466. [ Links ]
Sarkar, M., Niranjan, N., Banyal, P.K., 2017, Mechanisms of hypoxemia, Lung India 34(1), 47-60. https://doi.org/10.4103/0970-2113.197116. [ Links ]
Seddighi, R., Doherty, T.J., 2016, Field sedation and anesthesia of ruminants, The Veterinary clinics of North America, Food Animal Practice 32(3), 553-570. https://doi.org/10.1016/j.cvfa.2016.05.002. [ Links ]
Shah, Z., Mingxing, D., Manli, H., 2014, A review on the current use of a2-agonists in small ruminants, Kafkas Universitesi Veteriner Fakultesi Dergisi 20, 633-639. https://doi.org/10.9775/kvfd.2013.10541. [ Links ]
Sinclair, M.D., 2003, A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice, Canadian Veterinary Journal 44, 885-897. [ Links ]
Szabó, Z., Venter, D., Luyt, C., et al., 2015, The use of thiafentanil oxalate and azaperone for reversible immobilisation of African buffalo (Syncerus caffer) within a nature reserve - Short communication, Acta Veterinaria Hungarica 63(1), 11-15. https://doi.org/10.1556/avet.2015.002. [ Links ]
Vardanyan, R.S., Hruby, V.R, 2014, Fentanyl-related compounds and derivates: Current status and future prospects for pharmaceutical applications, Future Medicinal Chemistry 6 (4), 385-412. https://doi.org/10.4155/fmc.13.215. [ Links ]
Zeiler, G.E., Meyer, L., 2017, Comparison of thiafentanil-medetomidine to etorphine-medetomidine immobilisation of impalas (Aepyceros melampus), Journal of the South African Veterinary Association, 88(0), a1520. https://doi.org/10.4102/jsava.v88i0.1520. [ Links ]
 Correspondence:
Correspondence:
VE Faber
Email: vfaber87@gmail.com













