Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.83 n.1 Pretoria Jan. 2012
ORIGINAL RESEARCH
Technique for the collection of clear urine from the Nile crocodile (Crocodylus niloticus)
Jan G. MyburghI; Fritz W. HuchzermeyerI; John T. SoleyII; Dirk G. BooyseII; Herman B. GroenewaldII; Lizette C. BekkerI; Taisen IguchiIII; Louis J. Guillette, JrI, IV
IDepartment of Paraclinical Sciences, University of Pretoria, South Africa
IIDepartment of Anatomy and Physiology, University of Pretoria, South Africa
IIIDepartment of Bio-Environmental Science, National Institutes of Natural Sciences, Japan
IVDepartment of Zoology, University of Florida, United States of America
ABSTRACT
Urine samples can be a very useful diagnostic tool for the evaluation of animal health. In this article, a simple technique to collect urine from the Nile crocodile (Crocodylus niloticus) was described, based on a similar unpublished technique developed for the American alligator (Alligator mississippiensis) using a canine urinary catheter. With this technique, it was possible to collect relatively clean urine samples from Nile crocodiles of different sizes using canine urinary catheters or small diameter stomach tubes. Based on the gross anatomical features of the cloaca of the Nile crocodile, it was confirmed that urine accumulates in a chamber consisting of the urodeum and coprodeum. Faecal material is stored temporarily in the very short rectum, which is separated from the urinary chamber by the rectocoprodeal sphincter.
Introduction
Pollution is an escalating global problem.1,2,3,4 Chemicals and/or their metabolites, which are used as part of human activity, eventually become aquatic pollutants via run-off from plants and soil following agriculture application, sewage outflow, direct spraying of surface water to control pests, storm water runoff or after industrial or mining accidents.1,2,3 As such, anthropogenic pollution of rivers and lakes is fast becoming a major threat to aquatic biodiversity in southern Africa.5 Aquatic species are therefore considered to be valuable sentinels of pollution2,6 and crocodilians, as the top predators in aquatic ecosystems, are therefore excellent candidates for this purpose.2,7
Aquatic toxicologists studying sentinel animals such as crocodiles usually rely on blood samples from live animals8 or complete necropsies9 for etiological diagnostic purposes. Likewise, toxicokinetic and pharmacokinetic studies of non-mammalian species traditionally only use blood samples; yet, most contaminants and pharmaceutical agents are biotransformed to water-soluble metabolites by the liver and excreted in the urine.10,11 Considering that most crocodilians are listed as endangered or threatened, it is nearly impossible and ethically inappropriate to kill crocodilians for research purposes.12 Thus, minimally invasive techniques allowing researchers or veterinarians to obtain urine samples from crocodiles, in addition to blood, whether from wild or farmed crocodiles, would contribute significantly to our knowledge and future ability to assess the health status or to diagnose specific diseases in these animals.
With a few exceptions,13 existing descriptions of the lower digestive tract of crocodilians provide little detail on the morphology of the cloaca14,15 and it would appear that urine has been collected from crocodilians in the past without the collectors being acquainted with the morphology of this region.16,17,18,19,20 For example, more than eighty years ago, Hopping18 collected urine from the American alligator (Alligator mississippiensis) for analysis and stated that the cloaca 'acts as a bladder' because clear urine accumulates within it. More recently, Kuchel and Franklin13 described, in detail, the morphology and histology of the estuarine crocodile's (Crocodylus porosus) cloaca. However, their interpretation of the morphology led them to conclude that urine accumulates in the urodeum, whilst faecal material is stored separately in the coprodeum.13 No detailed description of the morphology and histology of the Nile crocodile's cloaca is currently available.
Urine has been collected previously from the American alligator,16,17,18 estuarine crocodile,13,21,22,23,24 Australian freshwater crocodile (Crocodylus johnsoni),25 Nile crocodile19,20,24 and the broad-snouted caiman (Caiman latirostris).26 Most of the collected urine samples were used for research projects that investigated osmoregulation in crocodilians.13,20,21,22,23,24,25,26 Leslie and Spotila20 collected urine from Nile crocodiles by inserting a blunt pair of stainless steel forceps through the cloacal opening. The technique used by Hopping18 is described as catheterisation of the cloaca, but no detail is given. Schmidt-Nielsen and Skadhauge27 used a relatively complicated method of collecting urine directly from the urinary papillae (urethral openings). However, it appears that the technique used most frequently by different researchers16,17,20-22,23,25-26 is to insert a glass tube or any other suitable instrument through the cloacal opening or vent. By gently forcing these instruments forward, the uroproctodeal sphincter is opened, allowing urine to flow freely through the proctodeum and into a sample bottle.
This paper describes a simple and non-traumatic technique for the collection of urine directly from the urinary chamber of the Nile crocodile by using a canine urinary catheter or a small diameter stomach tube. It is based on a procedure developed for use in the American alligator (Guillette LJ Jr and Iguchi T, personal communication, n.d.). Several catheters and stomach tubes were evaluated during this investigation to determine which would be most suitable. The paper also illustrates and discusses the gross morphology of the cloaca of the Nile crocodile, as related to this clinical procedure.
Materials and methods
Cloacal morphology
Ten juvenile farmed Nile crocodiles were selected during routine necropsy examinations and split sagitally along the midline to expose the cloaca in situ. An additional 10 juvenile farmed Nile crocodiles (obtained from Izintaba Crocodile Farm, South Africa) were euthanised by injecting sodium pentobarbitone (Eutha-naze, Bayer Animal Health, Isando, South Africa) intravenously into the post-occipital sinus of the spinal vein. The cloacae of these animals were filled with coloured latex introduced via the cloacal opening using a syringe. The caudal part of each carcass was separated, just cranial to the hind limbs, and stored in 10% neutral-buffered formalin for one week. Following fixation, the specimens were divided longitudinally along the midline to allow examination of the different latex-filled chambers of the cloacae. Additionally, the cloacae of five slaughter-size crocodiles (obtained from Izintaba Crocodile Farm, South Africa) were removed from the carcasses, after these animals were euthanised by injecting sodium pentobarbitone (Eutha-naze, Bayer Animal Health, Isando, South Africa) intravenously into the post-occipital sinus of the spinal vein. These cloacae were fixed in situ by filling them with 10% neutral-buffered formalin via the terminal ileum, having sealed the vent using a cork stopper. The cloacae were carefully dissected free of the surrounding tissues and split lengthwise to allow examination of the different chambers. Relevant gross anatomical features were described and digitally recorded.
Urine collection technique
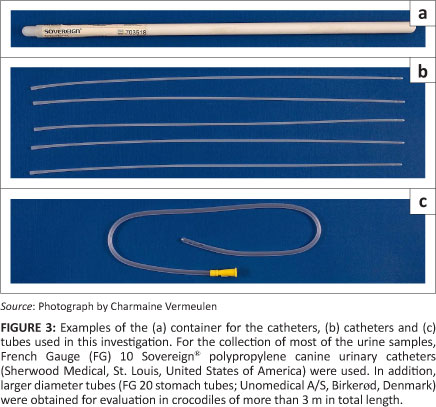
Canine urinary catheters (Smiths Medical International Ltd, Ashford, United Kingdom) of different sizes (French Gauge [FG] 6, 8 and 10) were obtained from the Onderstepoort Veterinary Academic Hospital's pharmacy to evaluate their suitability for the collection of urine from crocodiles. In addition, a large number of FG 8 and 10 Sovereign®; polypropylene canine urinary catheters (Sherwood Medical, St. Louis, United States of America) were donated to the Nile crocodile urine project by one of the co-authors (LJ Guillette).
Tubes with larger diameters were also obtained from the Onderstepoort Veterinary Academic Hospital's pharmacy for evaluation, namely FG 12 AvacareTM nelaton catheters (Well Lead Medical Co. Ltd, Guangzhou, China) and FG 20 stomach tubes (Unomedical A/S, Birkerad, Denmark). These larger diameter tubes were used specifically for evaluation in crocodiles of more than 3 m in total length (TL).
The various catheters and tubes were evaluated for suitability during urine collection28 from farmed (n > 130; mostly pre-slaughter crocodiles from Izintaba Crocodile Farm, South Africa) and wild (n = 18; mostly mature crocodiles, > 2.5 m in TL, from the Olifants River in the Kruger National Park) crocodiles of different sizes (> 0.5 m to < 4.2 m TL) to determine the most suitable and practical catheters or tubes to be used.
Ethical considerations
This project was approved by the Animal Use and Care Committee at the University of Pretoria, under the following project numbers: V040/05 and V041/05.
Results
Examination of cloacal morphology
Observations on the fresh post-mortem material of the juvenile Nile crocodiles presented inconclusive evidence on the compartmentalisation of the cloaca. In these specimens, three obvious chambers were observed, namely, (1) a caudal chamber (proctodeum) housing the phallus or clitoro-phallus, (2) a smooth-walled middle chamber with fine longitudinal folds containing yellow crystalline material and (3) a cranial chamber with transverse folds that contained a faecal pellet. However, examination of the cloacal material prepared in situ by filling with latex provided the first evidence that the apparently single chamber, located between the proctodeum and the chamber containing a faecal pellet, was subtly subdivided into two components, a caudally situated urodeum displaying the urinary papillae and a cranially situated coprodeum separated from the urodeum by a poorly developed fold, the coprourodeal fold (Figure 1). The cloacae prepared in situ by filling with fixative confirmed the findings in the latex-filled specimens.
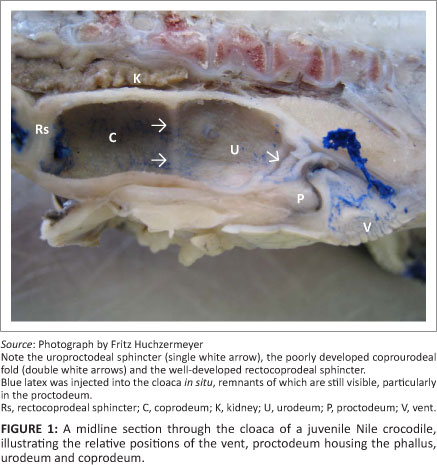
Based on these observations, it was determined that the cloaca consisted of three compartments, the proctodeum, urodeum and coprodeum (Figure 1). The most caudal compartment, the proctodeum, was the smallest and communicated with the exterior via a slit-like vent or cloacal opening. The phallus or clitoro-phallus lay ventrally in the proctodeum in male and female crocodiles, respectively. The proctodeum was separated from the urodeum by a distinct fold, the uroproctodeal sphincter. The urodeum formed the middle compartment of the cloaca and appeared to be continuous with the cranial compartment, the coprodeum, being separated in some specimens only by a poorly developed coprourodeal fold. Where this separation was visible, the urodeum was shorter in length than the coprodeum (Figure 1), although both compartments were approximately of equal width in juvenile animals. Two separate papillae were visible on the dorsal aspect of the posterior part of the urodeum, indicating the entry of the ureters into the compartment. The urodeum and coprodeum therefore formed a combined compartment (urinary chamber) in which the urine accumulates. Full urinary chambers were observed in several Nile crocodiles during necropsies.
Cranial to the coprodeum is the short rectum. It is demarcated clearly by a well-developed sphincter, from both the coprodeum (by the rectocoprodeal sphincter) (Figure 1) and the terminal ileum (by the ileorectal sphincter). When present, faecal material was always confined to the rectum. Chiasson14 preferred to use the term ileocolic sphincter for the sphincter between the ileum and the chamber containing the faecal pellet.
In Figure 2, the morphology of the Nile crocodile's cloaca is illustrated schematically, demonstrating the most important gross anatomical features that are of relevance to the urine collector.
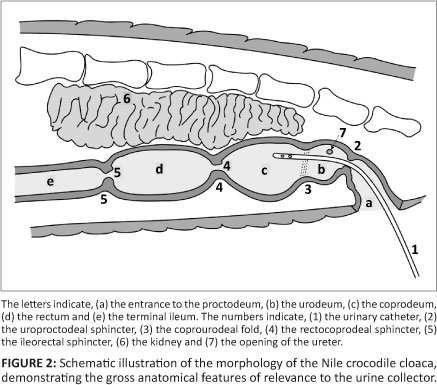
Urine collection technique
After evaluating different sizes of catheters and tubes, it was found that a FG 10 canine urinary catheter was the most suitable for the collection of urine from all sizes of crocodiles. For the collection of most of the urine samples,28 the FG 10 Sovereign®; polypropylene catheters (reference number: 8890703518) were used (Figure 3). However, for large crocodiles (> 3 m) larger diameter tubes were also tried. Unfortunately, the smaller of these tubes (FG 12) were not rigid enough and it was difficult to insert them through the uroproctodeal sphincter. The slightly larger diameter stomach tubes (FG 20) were rigid enough to be pushed through the sphincter (Figure 3). The advantage of the larger diameter tube (FG 20) is that urine flow is fast and a urine sample can be collected within seconds. When collecting urine samples from heavy, mature crocodiles it is not always easy to roll them over or, most often, impossible to lift them vertically. In such instances the advantage of the larger diameter tube is that whenever an opportunity is presented to insert the tube into the urinary chamber, urine collection will be completed very quickly.
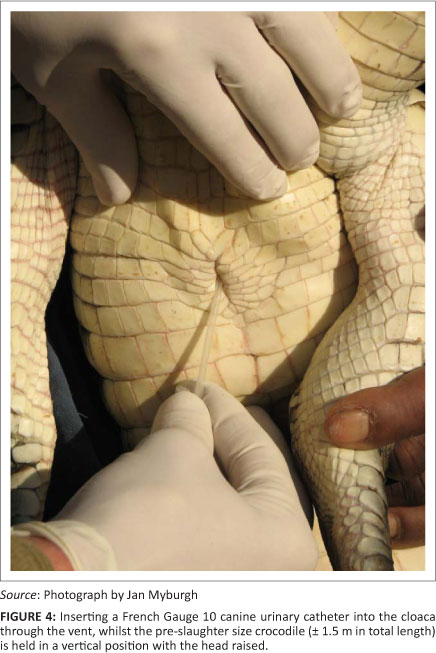
The catheter is inserted at the caudal end of the cloacal opening and pushed gently in a cranio-dorsal direction (Figure 4). The first obstruction during the passage of the catheter is the uroproctodeal sphincter (Figure 2). This sphincter is situated relatively close to the external cloacal opening and is approximately 2 cm - 4 cm from the opening in a 1.5 m crocodile. A slight obstruction is usually felt during the forward movement of the tip of the catheter, as it moves through the sphincter, but little resistance is generally encountered. This sphincter is not very strong and it is relatively easy to thread the catheter through it. Crocodiles sometimes urinate spontaneously if this sphincter is stimulated excessively with the tip of the catheter or a larger diameter tube. This urine can be collected directly into a wide-mouth container, but it is usually contaminated with proctodeal contents and external cloacal matter.
The rectocoprodeal sphincter, between the rectum and coprodeum, is much stronger and it is impossible to get the tip of the catheter into the rectum during the urine collection procedure (Figure 1 and Figure 2). When the area around this sphincter is touched with the tip of the catheter, it sometimes stimulates abdominal muscle contractions. This response is similar to the response seen in other animals if the anus is touched and is a very good indication that the tip of the catheter is deep within the urinary chamber and touching the rectocoprodeal sphincter. This is also an indication that the tip of the catheter should be pulled back slightly to obtain urine, especially in crocodiles suspended vertically. If no urine starts to flow, especially after the tip of the catheter has been alternated slowly between a deeper and shallower position, it is most probable that the urinary chamber is empty. If the urinary chamber is full, urine flow is usually immediate following entry of the tip of the catheter through the uroproctodeal sphincter.
A practical point influencing the success of urine collection from crocodilians is the position of the animals during collection. In juvenile or small crocodilians, the animal can be held vertically, or almost vertically with the head up, to facilitate urine flow. It is more difficult to obtain urine if the animal is lying horizontally on a table or on the ground. For adult animals, the tail can be propped up on the bent knee of the researcher, after which the catheter is introduced into the cloaca in the manner described above. This technique is used frequently for the collection of urine from large American alligators > 3 m in TL (Lowers R and Guillette LJ, Jr, personal communication, n.d.). In this way, the tail and part of the pelvis of the crocodile can be lifted off the ground and the catheter or tube inserted through the cloacal opening in a cranial direction. Urine will then flow without difficulty. If it is impossible to position a crocodile as recommended (the whole animal in a vertical position or only the tail and pelvis raised), another method is to roll the crocodile halfway over into a lateral recumbent position and to insert the catheter or tube whilst the crocodile is kept on its side. For all these different positions, it is important to keep the specimen bottle lower than the urinary chamber to promote free flow of urine.
No faecal material has been collected or observed in the more than 750 urine samples collected from American alligators30 and Nile crocodiles24 using this technique. This was also confirmed by Hopping18, who collected urine from the American alligator with a catheter. The urine samples that were collected were not tested for bacterial contamination.
Discussion
Collecting urine from the Nile crocodile is a relatively easy procedure allowing for the more frequent use of urine as a diagnostic sample to assess the health of wild and captive crocodiles. The advantage of this collection technique is that a relatively 'clean' urine sample is easily obtained, as only a small part of the sterile catheter or tube comes into contact with the proctodeum and uroproctodeal sphincter; thus, contamination of the urine sample is most likely to be minimal.
The possibility of a routine technique for the collection of urine samples from crocodilians opens new fields for future research. Chemical pollutants, pharmaceutical drugs and steroid hormones or their breakdown products are excreted in the urine. Urine could therefore provide an additional or alternative sample that may be better than plasma for some analyses, such as in the detection of metabolised and water soluble pollutants and drugs.10,11
Crocodilian researchers should also consider seriously making use of urine as an indicator of endocrine system abnormalities. Excretion of steroid hormones or metabolites in urine has been well studied in humans.29 However, nothing has been reported for crocodilians. Urine steroid profiling in humans can be indicative of numerous endocrine dysfunctions, including reproductive and thyroid disorders, enzyme deficiencies or excesses, as well as hypocortisism and hypercortisism (including tumours of the adrenal gland).29 The envisaged investigation of urinary steroid profiles of crocodilians which live in reference and polluted aquatic ecosystems, is expected to confirm the diagnostic value of a urine sample to detect endocrine disruptive disturbances in these animals.
Anatomical descriptions of the crocodilian cloaca are, in general, very superficial, differently interpreted and contain confusing terminology. For example, Richardson, Webb and Manolis15 do not describe the morphology of the cloaca, but, in their stylised drawings, illustrate the colon positioned cranial to the cloaca and a distinct constriction between what appears to be the coprodeum and urodeum. Chiasson14 presents a similar representation of the relative positions of the colon (the term rectum is preferred for the short but distinct large intestine) and cloaca, noting that the rectum is separated from the small intestine by the ileocolic sphincter and from the cloaca by a 'thick anal sphincter'. In a detailed study of the estuarine crocodile, Kuchel and Franklin13 report that the three chambers of the cloaca are separated by 'tight muscular sphincters' and that the urodeum forms the largest chamber. In this species, the coprodeum is illustrated as being small.13 In marked contrast, it was clear from the present study that in the Nile crocodile the urodeum and coprodeum form a combined urinary chamber separated from each other by a rudimentary coprourodeal fold (Figure 1). The relative ease in obtaining urine samples from the Nile crocodile certainly is facilitated by the structural arrangement of the urodeum and coprodeum (Figure 2). Whether these significant differences in cloacal morphology represent species-specific peculiarities or reflect differences in interpretation will require further investigation.
The strict separation of urine from faeces in the cloaca of the Nile crocodile is very similar to that observed in the ostrich, which stores urine in the coprodeum.30,31,32 In the Nile crocodile, faeces is stored in the rectum and held back by a powerful sphincter (rectocoprodeal sphincter) separating the rectum from the urinary chamber. The separate storage of clean urine in the cloaca is shared by tortoises, which have a bladder-like chamber attached to the urodeum,33 crocodiles13,22 and ostriches30,31,32,34 and appears to be a primitive feature that was abandoned in most birds, but retained by ostriches. Further work is needed to expand the understanding of the evolution of these differences.30,32 However, further specialisation appears to have taken place in the cloaca of the ostrich, where the urine is stored in the ventrally sagging coprodeum, which can be bypassed during defaecation.30,32
Conclusion
The cloacal morphology of crocodilians and its physiological interpretation is currently very poorly understood. A thorough comparative investigation of crocodilian (crocodilidae, alligatoridae and gavialidae) cloacal morphology and histology is, therefore, urgently needed. The physiological role of the coprodeum and urodeum in changing the post-renal composition of urine should also be investigated.
From this study, it was determined that it is possible to collect a relatively clean urine sample from the Nile crocodile with a canine urinary catheter or small diameter stomach tube. Urine accumulates in a chamber consisting of the urodeum and coprodeum. A urine sample, in addition to a blood sample, could be very helpful in assessing the health status of crocodiles.
Acknowledgements
Mr Pit Süssmann, the managing director of Izintaba Crocodile Farm, is thanked for his assistance, as are Prof. Christo Botha and Dr Johan Steyl from the Department of Paraclinical Sciences at the University of Pretoria, who were always willing to help. This project was funded by the Norwegian Council for Higher Education's Program for Development, Research and Education.
This paper is dedicated to Dr Herbert Penzhorn, a crocodile farmer from South Africa who was killed by his own crocodiles during an accident in February 2011.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors' contributions
The initial idea and the planning of this project were undertaken by L.J.G. (University of Florida), J.G.M. (University of Pretoria) and T.I. (National Institutes of Natural Sciences, Japan). J.G.M. (University of Pretoria), F.W.H. (University of Pretoria), D.G.B. (University of Pretoria), J.T.S. (University of Pretoria) and L.C.B. (University of Pretoria) did the clinical investigations and cloacal dissections. J.T.S. (University of Pretoria), H.B.G. (University of Pretoria), F.W.H. (University of Pretoria) and J.G.M. (University of Pretoria) interpreted the cloacal anatomy. J.G.M. (University of Pretoria), J.T.S. (University of Pretoria), F.W.H. (University of Pretoria) and L.J.G. (University of Florida) wrote the manuscript, whilst J.T.S. (University of Pretoria) edited the figures.
References
1. Colborn T, Dumanoski D, Myers JP. Our stolen future. New York: Dutton Books; 1996. [ Links ]
2. Guillette LJ, Jr, Crain DA, Gunderson MP, et al. Alligators and endocrine disrupting contaminants: A current perspective. Am Zool. 2000;40:438-452. http://dx.doi.org/10.1668/0003-1569(2000)040[0438:AAEDCA]2.0.CO;2 [ Links ]
3. Guillette LJ, Jr, Milnes MR. Recent observations on the reproductive physiology and toxicology of crocodilians. In: Grigg GC, Franklin CE, editors. Crocodilian biology and evolution. Chipping Norton: Surrey Beatty & Sons, 2000; p. 199-213. [ Links ]
4. Milnes MR, Bermudez DS, Bryan TA, et al. Contaminant-induced feminization and demasculinization of non-mammalian vertebrate males in aquatic environments. Environ Res. 2006;100:3-17. http://dx.doi.org/10.1016/j.envres.2005.04.002,PMid:15913597 [ Links ]
5. Bornman MS, Van Vuren HJ, Bouwman H, De Jager TC, Genthie B, Barnhoorn EJ. The use of sentinel species to determine the endocrine disruptive activity in an urban nature reserve. Report to the Water Research Committee, Pretoria, South Africa. Pretoria: Water Research Committee; 2007. [ Links ]
6. Colborn T, Thayer K. Aquatic ecosystems: Harbinger of endocrine disruption. Ecol Appl. 2000;10:949-957. [ Links ] http://dx.doi.org/10.1890/1051-0761(2000)010[0949:AEHOED]2.0.CO;2
7. Pooley T. Discoveries of a crocodile man. Johannesburg: William Collins Sons & Co Ltd; 1982. [ Links ]
8. Hamlin HJ, Lowers RH, Guillette LJ, Jr. Seasonal androgen cycles in adult male American alligators (Alligator mississippiensis) from a barrier island population. Biol Reprod. 2011;85:1108-1113. [ Links ]
9. Huchzermeyer FW. Crocodiles: Biology, husbandry and diseases. Wallingford: CABI Publishing; 2003. http://dx.doi.org/10.1079/9780851996561.0000 [ Links ]
10. Larsen JC. Levels of pollutants and their metabolites: Exposures to organic substances. Toxicology. 1995;101:11-27. http://dx.doi.org/10.1016/0300-483X(95)03017-A [ Links ]
11. McClellan-Green P, Celander M, Oberdórster E. Hepatic, renal, and adrenal toxicology. In: Gardner SC, Oberdórster E, editors. Toxicology of reptiles. Boca Raton: CRC Press, Taylor & Francis Group, 2006; p. 123-148. [ Links ]
12. Irwin L, Irwin K. Global threats affecting the status of reptile populations. In: Gardner SC, Oberdórster E, editors. Toxicology of reptiles. Boca Raton: CRC Press, Taylor & Francis Group, 2006; p. 9-34. [ Links ]
13. Kuchel LJ, Franklin CE. Morphology of the cloaca in the estuarine crocodile, Crocodylus porosus, and its plastic response to salinity. J Morphol. 2000;245:168-176. http://dx.doi.org/10.1002/1097-4687(200008)245:2<168::AID-JMOR7>3.0.CO;2-1 [ Links ]
14. Chiasson RB. Laboratory anatomy of the alligator. Dubuque: WMC Brown Company Publishers; 1963. [ Links ]
15. Richardson KC, Webb GJW, Manolis SC. Crocodiles: Inside out, a guide to the crocodilians and their functional morphology. Baulkham Hills: Surrey Beatty & Sons; 2002. [ Links ]
16. Coulson RA, Hernandez T. Alligator metabolism - Studies on chemical reactions in vivo. Comp Biochem Physiol B. 1983;74B:1-182. [ Links ]
17. Herbert JD. Nitrogen excretion in 'maximally-fed' crocodilians. Comp Biochem Physiol B. 1981;69B:499-504. [ Links ]
18. Hopping A. Seasonal changes in the gases and sugar of the blood and nitrogen distribution in the blood and urine of the alligator. Am J Physiol. 1923;66:145-163. [ Links ]
19. Khalil F, Hagag G. Nitrogenous excretion in crocodiles. J Expl Biol. 1958;35:552-555. [ Links ]
20. Leslie AJ, Spotila JR. Osmoregulation of the Nile crocodile, Crocodylus niloticus, in Lake St. Lucia, Kwazulu/Natal, South Africa. Comp Biochem Physiol A. 2000;126A:351-365. [ Links ]
21. Grigg GC. Plasma homeostasis and cloacal urine composition in Crocodylus porosus caught along a salinity gradient. J Comp Physiol B. 1981;144B:261-270. [ Links ]
22. Kuchel LJ, Franklin CE. Kidney and cloaca function in the estuarine crocodile (Crocodylus porosus) at different salinities: Evidence for solute-linked water uptake. Comp Biochem Physiol A. 1998;119A:825-831. [ Links ]
23. Taplin LE. Sodium and water budgets of the fasted estuarine crocodile, Crocodylus porosus, in sea water. J Comp Physiol B. 1985;155B:501-513. http://dx.doi.org/10.1007/BF00684681 [ Links ]
24. Taplin LE, Loveridge JP. Nile crocodiles, Crocodylus niloticus, and estuarine crocodiles, Crocodylus porosus, show similar osmoregulatory responses on exposure to seawater. Comp Biochem Physiol A. 1988;89A:443-448. http://dx.doi.org/10.1016/0300-9629(88)91054-7 [ Links ]
25. Taplin LE, Grigg GC, Beard LA, Pulsfords T. Osmoregulatory mechanisms of the Australian freshwater crocodile, Crocodylus johnstoni, in freshwater and estuarine habitats. J Comp Physiol B. 1999;169B:215-223. http://dx.doi.org/10.1007/s003600050214 [ Links ]
26. Grigg GC, Beard LA, Moulton T, Queirol Melo MT, Taplin LE. Osmoregulation by the broad-snouted caiman, Caiman latirostris, in estuarine habitat in southern Brazil. J Comp Physiol B. 1998;168B:445-452. [ Links ]
27. Schmidt-Nielsen B, Skadhauge E. Function of the excretory system of the crocodile (Crocodylus acutus). Am J Physiol. 1967;212:973-980. [ Links ]
28. Myburgh JG, Huchzermeyer FW, Bekker LC, et al. Farm and wild Nile crocodile (Crocodylus niloticus) plasma and urine chemistry investigation. In preparation 2012. [ Links ]
29. Bekker LC, Bester M, Hurter P. Steroid profiles in 0 to 3 day old neonates. J Microcolumn Sep. 2001;13:250-254. http://dx.doi.org/10.1002/mcs.1050 [ Links ]
30. Laverty G, Skadhauge E. Adaptive strategies for post-renal handling of urine in birds. Comp Biochem Physiol A. 2008;149A:246-254. [ Links ]
31. Skadhauge E. Osmoregulation in ratite birds: Kidney-gut interactions in excretion of electrolytes and water in ostrich, emu and rhea. In: Huchzermeyer FW, editor. Proceedings of the 2nd International Ratite Congress; 1998 Sept 21-25, Oudtshoorn, South Africa. Cape Town: De Jongh Printers, 1998; p. 84-87. [ Links ]
32. Warui CN, Skadhauge E. Morphological and functional anatomy of the cloaca and terminal colon of the African ostrich. In: Huchzermeyer FW, editor. Proceedings of the 2nd International Ratite Congress; 1998 Sept 21-25, Oudtshoorn, South Africa. Cape Town: De Jongh Printers, 1998; p. 88-90. [ Links ]
33. Khalil F, Hagag G. Ureotelism and uricotelism in tortoises. J Exp Zool. 1955;130:423-432. http://dx.doi.org/10.1002/jez.1401300304 [ Links ]
34. Skadhauge E. Excretion in lower vertebrates: Function of gut, cloaca, and bladder in modifying the composition of urine. Fed Proc. 1977;36:2487-2492. [ Links ]
 Correspondence to:
Correspondence to:
Jan Myburgh
Private Bag X04
Onderstepoort 0110
South Africa
jan.myburgh@up.ac.za
Received: Feb. 2008
Accepted: Feb. 2012
Published: 04 July 2012













