Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 n.1 Pretoria Jan. 2010
ARTICLE ARTIKEL
Retrospective study on the incidence of Salmonella isolations in animals in South Africa, 1996 to 2006
A KidanemariamI,*; M EngelbrechtI; J PicardII
IOnderstepoort Veterinary Institute, Agricultural Research Council, Private Bag X05, Onderstepoort, 0110 South Africa
IIFaculty of Veterinary Sciences, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
ABSTRACT
A retrospective study that involves the analysis of laboratory diagnostic data collected during the period 1996-2006 was conducted. A total of 3417 Salmonella isolations involving 183 different serotypes was recorded from 1999-2006, inclusive, at the Onderstepoort Veterinary Institute, Agricultural Research Council, South Africa. The most common serotypes were Salmonella enterica subspecies enterica serovar Typhimurium (917 incidents), Salmonella enterica subspecies enterica serovar Dublin (248 incidents), Salmonella enterica subspecies enterica serovar Enteritidis (232 incidents), Salmonella enterica subspecies enterica serovar Muenchen (164 incidents), Salmonella enterica subspecies enterica serovar Heidelberg (118 incidents) and Salmonella enterica subspecies enterica serovar Chester (113 incidents). The number of recorded Salmonella isolations over the period 1996 to 2006 varies considerably from year to year. The peak of 693 isolations was recorded in 1997, and the lowest, 108 incidents, in 2001. Of the total incidents recorded during the period of survey, 2410 (70.5 %) occurred in poultry and other birds, 641 (18.75 %) occurred in cattle, 255 (7.46) in pigs and 111 (3.24 %) in sheep. Despite the large number of serotypes isolated (183), 52 % of incidents were due to only 6 serotypes in decreasing order of prevalence: S. Typhimurium, S. Dublin, S. Enteritidis, S. Muenchen, S. Heidelberg and S. Chester. Serovar Typhimurium was the most common serotype and was detected in all animal species sampled, with, 65 % (598) of the incidents occurring in poultry and 20 % (187) occurring in cattle. Of the total of 248 incidents of S. Dublin serotype, 95.6 % (237) of incidents occurred in cattle and of the 232 isolates of S. Enteritidis, 223 (96%) originated from poultry. Serovar Choleraesuis was identified in 16 isolates from pigs. The following 4 serotypes were each recorded in more than 50 incidents: S. Hadar (102), S. Schwarzengrund (99), S. Mbandaka (94) and S. Sandiego (73). The trends of annual incidence of Salmonella infection in cattle, sheep, pigs, poultry and other birds during the 11-year period and the distribution of the main serotypes in individual species of animals from 1996-2006 are discussed.
Keywords: incidence, livestock, Salmonella enterica, South Africa.
INTRODUCTION
Salmonellosis in farm animals and its association with human infection has attracted increasing attention in many countries4,,24,28,33. It is well recognised that the incidence of Salmonella infection in various species of farm animals is closely linked with husbandry methods and that intensive farming methods are conducive to the spread of infection and a resulting rise in clinical disease. In fact, in many countries such as Sweden, salmonellosis is a controlled disease where especially poultry farms are routinely monitored for the presence of Salmonella species and if present, measures are taken to eradicate the agent3. Using these programmes, the incidence of salmonellosis in a given species of animal can be reduced to a level at which it is no longer of economic or clinical importance, provided that satisfactory diagnostic methods are available and appropriate control measures are instituted based on the diagnostic test results. This is well illustrated by the reduction in the incidence of S. Pullorum and S. Gallinarum infection in the European Union, resulting from the compulsory blood testing programme in poultry establishments3.
In South Africa, S. Enteritidis, and since 2008 S. Gallinarum are notifiable diseases. In spite of this, many farms, especially poultry farms, are monitoring for salmonellae and taking measures to eradicate the agents. Salmonellosis in cattle in South Africa is frequently diagnosed in calves, but it is only rarely encountered as a clinical problem in adult cattle28. Although an accurate estimate of the occurrence of salmonellosis in cattle in southern Africa is not available, its importance is likely to be similar to that in many other parts of the world17. Although present in pigs, salmonellosis is generally considered by this industry to be of lesser importance compared with the other diseases. Clinical salmonellosis in pigs may, however, become economically important in South Africa as the prevalence of porcine circovirus-2 increases1. Furthermore, multiresistant S. Typhimurium strains that are potentially of public health significance have been identified from pigs 26.
Non-typhoid salmonellosis is by far the most frequently reported food-borne zoonotic disease across the globe. Epidemiological investigations have shown that poultry, poultry products, pork and raw milk are the most common sources of sporadic outbreaks of human salmonellosis19,20,32.
The Bacteriology Laboratory at the Onderstepoort Veterinary Institute (ARC-OVI) is regarded as the referral laboratory for the serotyping of salmonellas of animal origin in South Africa. It therefore receives the vast majority of salmonellae of animal origin for serotyping from provincial and private veterinary laboratories. Some salmonellae are, however, cultured and typed at other laboratories and are not included in this survey.
The purpose of this review is therefore to present retrospective laboratory serotyping data from the ARC-OVI between 1996 and 2006 in livestock in South Africa.
MATERIALS AND METHODS
Samples and laboratory serotyping
The Bacteriology Laboratory of ARC-OVI receives Salmonella strains for serotyping from all provinces in the country. The laboratory also performs the isolation and identification of Salmonella species. The bacterial strains were isolated from animal and non-animal sources. Only those Gram-negative strains that were indolenegative, motile, Simmond's citrate-positive, urease negative, produced hydrogen-sulphide in a triple sugar iron (TSI) slant, lysine decarboxylase positive, fermented dulcitol but did not ferment lactose and were malonate negative were considered to be Salmonella enterica9 and were serotyped. Exceptions were S. Pullorum and S. Gallinarum which are non-motile.
Serotyping of the Salmonella species was done by the slide agglutination test using polyvalent and monovalent antisera raised against the somatic (O) antigen and flagellar (H) antigen (BioRad PLC, Davies, PLC). Serovar identification was done by comparing the antigenic formula of strains with those described in the Kauffmann-White Scheme22.
Data management
All relevant diagnostic data generated by the laboratory were recorded in a data-capturing format and entered into a Microsoft 2003 ExcelTM spreadsheet for subsequent analysis. Descriptive statistics were employed to analyse the proportions of each serovar related to every animal species.
RESULTS
Total incidence of salmonella infection in different species of animals during the 11-year period 1999-2006 inclusive
The total number of incidents and distribution of various Salmonella serotypes in cattle, pigs, sheep, poultry and other birds, in South Africa are shown in Table 1. The 9 most common serotypes are listed in order of frequency, followed by the remainder in alphabetical order.
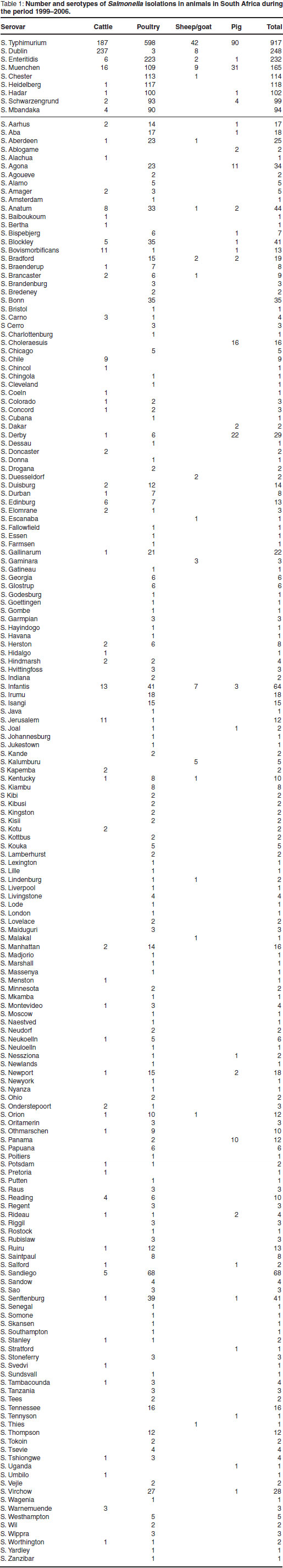
Of a total of 3417 incidents reported during this period and associated with 183 different serotypes, 2410 occurred in poultry and other birds (155 serotypes), 641 in cattle (66 serotypes), 255 in pigs (32 serotypes) and 111 in sheep (22 serotypes).
Despite the large number of serotypes isolated, the majority of incidents were due to relatively few serotypes. Moreover, with the exception of S. Typhimurium, which accounted for 917 incidents in all species of animals, the other commonly occurring serotypes had a relatively high degree of host specificity. Thus S. Dublin, the cattle-adapted serotype, was by far the most common serotype encountered in cattle (237 incidents), but was also rarely isolated in sheep (8 incidents). Serovar Enteritidis and S. Gallinarum were, with a few exceptions, confined to birds. All the serovar Choleraesuis strains originated from pigs.
Other serotypes frequently reported, namely S. Muenchen (165 incidents), S. Heidelberg (118 incidents), S. Chester (114 incidents), S. Hadar (102 incidents), S. Mbandaka (94 incidents) and S. Schwarzengrund (93 incidents) were
found mainly in poultry and other birds.
Of the total number of incidents reported in all species of animals, 26.8 % were due to S. Typhimurium infection (917 incidents), 7.3 % to S. Dublin (248 incidents), 6.8 %to S. Enteritidis (232 incidents), 4.8 % to S. Muenchen (165 incidents), 3.3%to S. Chester (114 incidents), 3.4%to S. Heidelberg (118 incidents), 2.9%to S. Hadar (102 incidents), 2.7 % to S. Mbandaka (94 incidents), 2.9 % to S. Schwarzengrund (99 incidents), 2.1 % to S. Sandiego (73 incidents), 1.8 % to S. Infantis (64 incidents), 1.3 % to S. Anatum (44 incidents) and 1.2 % to S. Blockley (41 incidents).
The remaining 170 serotypes (1106 incidents) collectively accounted for 32.3 % of the total incidents.
The annual incidence of Salmonella strains in cattle, sheep, pigs and poultry is shown in Table 2, which indicates a slight increase in the incidence of infection in cattle and similarly slight decrease in the incidence in poultry. There was a slight increase in the number of incidents in sheep from year to year.
The data in Table 2 show that the total number of Salmonella incidents reported in 1997 increased by 206 % compared with 1996 and increased by 170 % compared with 2005. However, this was not consistent throughout the survey period. The proportion of Salmonella incidents in 2001 was low and accounted for only3%of the total reported incidents. Over the 11-year survey period, Salmonella incidents reported were significantly higher in avian species than the other species. The incidents in avian species accounted for 70.5 % of the total incidents reported during the same period.
Salmonella infection in cattle
The main features of the annual incidence of Salmonella infection in cattle are presented in Table 4.
Of a total of 641 incidents of Salmonella infection, 36.9 % were due to S. Dublin (237 incidents) and 29 % to S. Typhimurium (187 incidents). Thus 66 % of all incidents were due to these 2 serotypes. Sixtyfour other serotypes accounted for the remaining 34 % of incidents (Table 4).
The highest number of incidents in cattle occurred in 2006 (Table 1). This was due to a dramatic rise in S. Typhimurium incidents (Table 4) to 51 (65 %), the highest number ever recorded since 1996. Since 1996 the annual incidence has declined to 30 (4.7 %) in 2001. This is partly due to the lower incident rate of S. Typhimurium recorded (2.2 %). However, in 2006 S. Typhimurium incidents reached a level of 51 (27.3%), the highest recorded for the period investigated.
Salmonella infection in poultry
The term 'poultry' includes domestic fowl, turkeys and ostriches. In all, 2410 incidents of Salmonella infection in poultry were diagnosed during 1996-2006 at the OVI (Table 5). Five hundred and ninety-eight incidents (24.8 %) were due to S. Typhimurium. Next in order of frequency was S. Enteritidis, with 223 incidents (9.3 %), followed by S. Heidelberg with 117 incidents (4.8 %), S. Chester with 113 (4.7%),) S. Muenchen with 109 (4.5 %), and S. Hadar with 100 (4.2 %). One hundred and forty-eight other serotypes accounted for the remaining 1150 cases (47.7 %).
Serovar Typhimurium was listed as the most common serotype (24.8 % of the total incidents) in this survey. The percentage of incidents due to this serotype varied from 43.5% in 1999 to 38.5 % in 2002; during 2006, 7.4 % incidents were recorded.
Serovar Enteritidis was second in frequency of isolation during the present survey (223 incidents) and the proportion of incidents due to this serotype varied from 11 % in 1996 to 23.7 % in 1998; during 2006, 5.9 % incidents were recorded.
The annual incidence of Salmonella infection rose significantly from 263 in 1996 to 625 in 1997 (see Table 5), but thereafter there was no regular trend in the annual incidence, largely due to irregular patterns in the isolation of the most common Salmonella serovars.
Salmonella infection in pigs
The incidence of Salmonella infection in pigs during the 11-year period is presented in Table 6. Although salmonellosis, especially S. Choleraesuis infection, can be a serious problem in pigs, its incidence was relatively low. Of the 255 incidents diagnosed during this period 90 (35.3 %) were due to S. Typhimurium, 31(12.2%) to S. Muenchen, 22 (8.6%) to S. Derby and 16 (6.3 %) to S. Choleraesuis. The remaining 96 incidents (37.6 %) were distributed between 28 other serotypes. Annual variations in incidents were mainly due to changes in the incidence of S. Typhimurium as this was by far the most common serotype involved. Although S. Muenchen was the second in frequency of isolation (22 incidents), it was reported on only 2 occasions during the survey period.
Salmonella infection in sheep
A total of 111 incidents of Salmonella infection in sheep, involving 21 serotypes, were reported during the 11-year period of the present survey. The main features are shown in Table 7. Of the 111 incidents diagnosed during this period 42 (37.8 %) were due to S. Typhimurium, 9(8.1%) to S. Muenchen, 8(7.2%) to S. Dublin and 7(6.3 %) to S. Infantis. The remaining 45 incidents (40.5 %) were distributed between 17 other serotypes. The percentage of infections in sheep due to serotypes other than the above has increased markedly from less than 25 % in 2003 to nearly 62 % in 2005. During 2006, these serotypes accounted for 47.6 % of the total incidents.
DISCUSSION
Since there is no coordinated effort to survey animals in South Africa for the presence of Salmonella, laboratory reports based on samples from diseased animals, abattoir and animal feed surveillance will continue to provide important information on the presence of Salmonella in livestock in this country. As this laboratory serves as a referral centre to all the State-run veterinary laboratories and is also used by many private laboratories for the serotyping of salmonellae, it gives a good indication of the prevalence of Salmonella infections in livestock in South Africa.
Over 2500 Salmonella serotypes are now internationally recognised8,21 and the number continues to increase every year. However, despite the existence of such a formidable number of different serotypes, only a few of these are frequently associated with clinical diseases in animals and humans31. Those salmonellae that are considered to be highly host-adapted, i.e. S. Dublin in cattle, and S. Gallinarum and S. Pullorum in chickens tend to cause clinical disease in their host-species, but only rarely in other species. However, other serovars such as S. Enteritidis in poultry and S. Choleraesuis in pigs tend to easily infect other species and are occasionally responsible for infections in humans and other animals2. The ubiquitous species, S. Typhimurium, infects a wide host range and is the most common serovar isolated in human non-typhoidal infections, especially humans infected with human immunodeficiency virus (HIV)15.

Despite the fact that more than 180 serotypes were characterised in this laboratory, the majority of incidents were due to only a few serotypes: 13 serotypes were responsible for 67.7 % of the total incidents (Table 1). Serovar Typhimurium was by far the most common, being recorded in 917 incidents (representing 26.8 % of the total), the majority of which occurred in poultry (598 incidents) in which it accounted for 65 % of the incidents. It was also the most common serotype reported in cattle (187 incidents), pigs (90 incidents) and sheep (42 incidents). As expected, the host-adapted serotypes such as S. Dublin, S. Choleraesuis and S. Enteritidis were almost exclusively isolated from cattle, pigs and poultry respectively. The low numbers of S. Gallinarum isolated from chickens was unusual but not unexpected. Biotyping methods identified these bacteria as S. Gallinarum and not S. Pullorum. Serovar Gallinarum is a common cause of septicaemia in layers and is frequently isolated by laboratories specialising in poultry diagnostics (J Picard, pers. obs., 2007), and is usually identified based on biochemical tests. At this stage it should also be noted that some laboratories have a limited capacity to serotype their own Salmonella strains and therefore many will identify common serotypes, but will not identify the unusual ones. The true country prevalence figures for S. Typhimurium, S. Dublin, S. Choleraesuis and S. Enteritidis may even be higher than presented here.
The present survey, covering 11 years from 1996 to 2006, shows more or less similar proportions of salmonella isolations in animals in South Africa (see Table 2). A notable exception is the proportion of Salmonella isolation in 1997 which reached the highest peak of 693, which is a 2fold increase from 1996 during which only 336 incidents were recorded. This increase in the total incidence was chiefly due to a rise in the number of incidents in poultry due to S. Typhimurium (168 incidents), S. Enteritidis (94 incidents) and S. Mbandaka (76 incidents) which altogether accounted for 338 reported incidents. One of the contributing factors to an increased incidence in the isolation of Salmonella serovars in poultry in the year 1997 may be linked to a policy shift in the country that stimulated the import of huge quantities of poultry products from abroad, and as the result there was a parallel increase in laboratory testing to ensure the safety and quality of the imported products (M Engelbrecht, pers. obs., 2008). There was also a slight peak in the isolation of salmonellae in poultry in 2005 and this could be attributed to the massive outbreak of velogenic Newcastle disease in poultry in South Africa as the result of which there may have been more chickens presented for necropsy and culture (J Picard, pers. obs., 2005). Often, when there are high mortalities in any species, the incentive to look for possible causal sources other than the main cause would also increase. It is also noted that there was a slight decrease in the number of Salmonella isolations in 2000 and 2001. This may be associated with a laboratory crisis rather than a genuine decrease in prevalence. However, care must also be taken when comparing data from one year with another as an increase or decrease in the number of isolations does not necessarily indicate a similar change in prevalence. This is because the total number of cases examined and their distributions are not known.
Serovar Dublin and S. Typhimurium are the 2 predominant serotypes detected in cattle, accounting for 66 % of the total recorded incidents. It has been shown elsewhere that S. Dublin is the most frequently isolated Salmonella serotype in clinical cases in cattle 34. A similar study has also shown that S. Typhimurium is the 2nd most important serotype, after S. Dublin, involved in clinical outbreaks of salmonellosis in dairy herds in the Netherlands36.
The relative incidence of S. Dublin in cattle had been consistently higher than the incidence of S. Typhimurim until 2006, when the proportion of S. Typhimurium incidents to those due to S. Dublin changed to a ratio of about 3:1. Earlier work indicated that the incidence of one serotype compared to another may differ from time to time for various reasons27.It is considered that these differences could be due to greater use of the live S. Dublin vaccine and managerial policies resulting in the removal of suspect S. Dublin carriers. Furthermore, an increase in animals that received rations of stored feed or greater rodent populations may have contributed to the increase in S. Typhimurium strains being cultured6.
It should be noted that the epidemiology of S. Typhimurium and S. Dublin varies considerably. Cattle infected with S. Dublin invariably continue to excrete large numbers of organisms in their faeces for many years and often for life14,25. Removal of cattle chronically infected with this serotype is therefore a logical control measure following implementation of appropriate control strategies to limit environmental, water, and feed related pathogen spread28. The period of excretion of S. Typhimurium is usually limited to a few weeks or months after clinical recovery11. It is known that S. Typhimurium strains from cattle tend to persist in the environment for long periods. In Great Britain, phage typing was able to show that certain phage types of S. Typhimurium found in cattle can persist for years and then are later replaced by another phage type23.
Serovar Enteritidis was mainly isolated from poultry with the overall incidence rate of 9.3 % (223 incidents). This serotype is among the most common pathogens of chickens that could also adversely affect human health following exposure to infected or contaminated chicken products18,30. Furthermore it can also cause serious disease in other livestock species such as cattle and sheep.
Serovar Choleraesuis was reported in 16 incidents (6.3%), but it was confined to pigs in which it was the 4th commonest serotype. This porcine serotype tends to cause septicaemic disease in humans in Asia where there are high pig densities5.
Although the epidemiology of salmonellosis caused by different Salmonella serotypes can be vastly different, relatively more research has been done on risk factors for Salmonella species as a group than for the specific Salmonella serovars10,34. The presence of a wide range of Salmonella serotypes in animal foodstuffs and fertilisers has attracted much interest in recent years. Poultry manure and litter which are commonly used as feed supplements because of their high nitrogen content are one source of Salmonella infection in animals31. A laboratory result (OVI, unpubl. data 2007) showed that feed samples submitted for the detection of Salmonella species proved to contain Salmonella serotypes and it is possible that contaminated foodstuffs may be a source of infection to animals in South Africa.
Faecal waste represents the largest reservoir of Salmonella on animal farms. A number of studies have examined the survival of this bacterium in animal waste maintained under anaerobic and aerobic conditions12. During an outbreak and even on farms with endemic Salmonella infections, the prevalence of faecal shedding of this bacterium may approach 90 %16. This would explicitly indicate another possible source of Salmonella infection in livestock in South Africa, where dung and livestock enclosure wastes could be used as manure fertilisers.
The public health significance of these bacteria should never be underestimated. Previous studies conducted in Gauteng province in 2004 in chicken carcasses entering the human food chain showed the presence of Salmonella species35. Studies from other countries have also shown that outbreaks and individual cases of salmonellosis in humans are most frequently associated with food products of animal origin and include eggs, meat, and milk byproducts7,13 .
In conclusion, this laboratory based survey report is believed to give an insight into the overall dynamics and the rate of Salmonella isolations in livestock and poultry in South Africa, and will provide an impetus for further investigation into the epidemiology and risk factors of salmonellosis in animals as well as the risk associated with human health.
ACKNOWLEDGEMENTS
We owe special debt of gratitude to Drs A Michel, A Potts and S Njiro for their encouragement, full support and reviewing of the manuscript.
REFERENCES
1. Allan G M, Ellis J A 2000 Porcine circoviruses: a review. Veterinary Diagnostic Investigation 12:3-14. [ Links ]
2. Anderson R J, Walker R L, Hird D W, Blanchard P C 1997 Case-control study of an outbreak of clinical disease attributable to Salmonella infection in eight dairy herds. Journal of American Veterinary Medical Association 210:528-530 [ Links ]
3. Anon. 2000 White paper on food safety of 12 January 2000 (COM/99/0719). Commission of the European Communities, Brussels. [ Links ]
4. Bean N H, Griffin P M 1990 Foodborne disease outbreaks in the United States, 1973-1987: pathogens, vehicles, and trends. Journal of Food Protection, 53:804-817 [ Links ]
5. Chiu C H, Su L H, Chu 2004 Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease and treatment. Clinical Microbiology Reviews 17:311-322 [ Links ]
6. Coetzer J A W, Tustin R C 2004 Salmonella species infection. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock, Vol. 3. Oxford University Press, Cape Town: 1578-1581 [ Links ]
7. Cohen M L,Tauxe R V 1986 Drug resistant Salmonella in the United States: an epidemiologic perspective. Science 234:964-969 [ Links ]
8. Cooper G L 1994. Salmonellosis - infection in man and the chicken: pathogenesis and the development of live vaccines - a review. Veterinary Bulletin 64:123-143 [ Links ]
9. Ellermeier C D, Slauch J M 2006. The genus Salmonella. In Dworkin M, Falkow S, Rosenberg E, Schleifer KH., Stackebrandt E (Eds) Prokaryotes, Vol. 6. Springer Science & Business Media LLC, New York, USA: 123-158 [ Links ]
10. Evans S J, Davies R H 1996 Case-control study of multiple-resistant Salmonella typhimurium DT104 infection in cattle in Great Britain. Veterinary Record 139:557-558 [ Links ]
11. Fenwick S G, Collett M G 2004 Bovine salmonellosis. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock, Vol. 3. Oxford University Press, Cape Town: 1582-1593 [ Links ]
12. Findlay C R 1972 The persistence of Salmonella Dublin in slurry in tanks and on pasture. Veterinary Record 91:233-235 [ Links ]
13. Fontaine R, Cohen M L, Martin W L,Vernon T W 1980 Epidemic salmonellosis from cheddar cheese: surveillance and prevention. American Journal of Epidemiology 111:247-253 [ Links ]
14. Gitter M, Wray C, Richardson C, Pepper R T 1978 Chronic Salmonella Dublin infection in calves. British Veterinary Journal 134:113-121 [ Links ]
15. Gordon M 2008 Salmonella infections in immunecompromised adults. Journal of Infection 56:413-422 [ Links ]
16. Groothuis D G, Miert A V, Van-Miert A 1987 Salmonellosis in veal calves. Some therapeutic aspects. Veterinary Quarterly 9:91-96 [ Links ]
17. Hobbs B C 1974 Microbiological hazards of meat production. Food Manufacture, 49:29-31 [ Links ]
18. Kimura A C, Reddy V, Marcus R, Cieslak P R, Mohle-Boetani J C, Kassenborg H D 2004 Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clinical Infectious Diseases 38 (Suppl. 3): 244-252 [ Links ]
19. Maguire H C F, Codd A A, Mackay V E, Rowe B, Mitchell E 1993 A large outbreak of human salmonellosis traced to a local pig farm. Epidemiology and Infection 110:239-246 [ Links ]
20. Mishu B, Koehler J, Lee L A, Rodrigue D, Brenner F H, Blake P, Tauxe R V 2004 Outbreaks of Salmonella enteritidis infections in the United States, 1985-1991. Journal of Infectious Diseases 169:547-552 [ Links ]
21. Murry C J 1991 Salmonellae in the environment. Review scientific technique Office International Epizootics 10:765-785 [ Links ]
22. Popoff M Y 2001 Antigenic Formulas of the Salmonella Serovars (8th edn). WHO Collaborating Centre for Reference and Research on Salmonella. Institute Pasteur, Paris, France [ Links ]
23. Rabsch W, Andrews H L, Kingsley R A, Prager R, Tschäpe H, Adams L G, Bäumler A J 2002 Salmonella enterica Serotype Typhimurium and its host-adapted variants. Infection and Immunity 70.2249-2255 [ Links ]
24. Radostitis O M, Blood D C, Gary C C 1994. Veterinary medicine (8th edn). Baillière, Tindall, London [ Links ]
25. Rice D H, Besser T E, Hancock D D 1997 Epidemiology and virulence assessment of Salmonella Dublin. Veterinary Microbiology 56:111-124. [ Links ]
26. SANVAD 2007 South African National Veterinary Surveillance and Monitoring Programme for Resistance to Antimicrobial Drugs. University of Pretoria. ISBN: 978-86854-673-2 [ Links ]
27. Sojka W J, Wray C 1975 Incidence of Salmonella infection in animals in England and Wales, 1968-73. Veterinary Record 96:280-284 [ Links ]
28. Spier S J, Smith B P, Dilling G E 1993 Enzyme-linked immunosorbent assay for serologic detection of Salmonella Dublin carriers on a large dairy. American Journal of Veterinary Research 54:1391-1399 [ Links ]
29. Stadler P, Nesbit J W 1990 Salmonellosis in an adult dairy cow. Journal of the South African Veterinary Association 61:65-67 [ Links ]
30. St Louis M E, Morse D L, Potter M E, Demelfi T M, Guzewich J J, Tauxe R V 1988 The emergence of Grade A eggs as a major source of Salmonella enteritidis infections: implications for the control of salmonellosis. Journal of American Medical Association 259:2103-2107 [ Links ]
31. Warnick L D, Crofton L M, Pelzer K D, Hawkins M J 2001 Risk factors for clinical salmonellosis in Virginia, USA, cattle herds. Preventive Veterinary Medicine 49:259-275 [ Links ]
32. Wegener H C, Baggesen D L 1996 Investigation of an outbreak of human salmonellosis caused by Salmonella enterica ssp. enterica serovar Infantis by use of pulsed field gel electrophoresis. International Journal of Food Microbiology 32:125-131 [ Links ]
33. Wray C 1985 Is salmonellosis still a serious problem in veterinary practice. The Veterinary Record 116:485-489 [ Links ]
34. Vaessen M A, Velinj J H, Frankena K, Graat E A M, Klunder T 1998 Risk factors for Salmonella Dublin infection on dairy farms. Veterinary Quarterly 20:97-99 [ Links ]
35. Van Nierop W, Duse A G, Marais E, Aithma N, Thothobolo N, Kasse L M, Stewart R, Potgieter A, Fernandes B, Galpin J S, Bloomfield S F 2005 Contamination of chicken carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes and Campylobacter. International Journal of Food Microbiology 1(99)(1):1-6 [ Links ]
36. Veling J, Barkema H W, Van der Schans J, Van Zijderved F, Verhoeff J 2002 Herd-level diagnosis of Salmonella enterica subsp enterica serovar Dublin infection in bovine dairy herds. Preventive Veterinary Medicine 53:31-42 [ Links ]
Received: August 2009.
Accepted: February 2010.
* Author for correspondence. E-mail: kidanemariama@arc.agric.za













