Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151Print version ISSN 0038-2361
S. Afr. j. surg. vol.62 n.4 Cape Town 2024
https://doi.org/10.36303/SAJS.00708
GENERAL SURGERY
Outcomes of surgical patients in a tertiary ICU with incidental COVID-19 in comparison with COVID-19 naïve patients
M Parker; I Mia; N Ahmed; V van der Westhuizen; A Diayar; J Buitendag
Department of Surgery, Tygerberg Hospital, Stellenbosch University, South Africa
ABSTRACT
BACKGROUND: COVID-19 was first identified in Wuhan, China, in December 2019, where it spread over a wide geographic area until it reached the status of a pandemic in 2020. We postulated that patients who were diagnosed with incidental COVID-19, and underwent surgery, did not have a worse outcome due to the COVID-19 virus compared to their counterparts who did not have the virus.
METHODS: This retrospective study included surgical patients (COVID-19 incidentals and COVID-19 negatives) who were admitted to the surgical intensive care unit (SICU) at Tygerberg Academic Hospital between 1 May 2020 and 31 December 2021.
RESULTS: The sample consisted of 578 patients. Forty-one (41) patients had incidental COVID-19 infection, and 537 patients were COVID-19 naïve. The mean age was 43.9 years (SD = 16.7 years; range = 13.0-82.0 years) and 181 (31.3%) were female. The rates of complications in patients with COVID-19 infection (7.3%) and those without (5.0%) were similar (p = 0.64). Grades of complications, as measured using the Clavien-Dindo classification were also similar between patients with and without COVID-19 infection (p = 0.19). The mortality rates of patients with COVID-19 infection (17.1%) and those without (13.6%) were similar (p = 0.53).
CONCLUSION: This study demonstrates that surgery among asymptomatic PCR-positive patients was not associated with increased mortality or morbidity in the SICU. This also adds a valuable contribution to the growing body of literature regarding COVID-19 infections. Further prospective and multicentred studies are required to provide more robust results.
Keywords: incidence, COVID-19, outcomes, surgery, ICU
Background
The COVID-19 virus is an enveloped, non-segmented, positive sense single-stranded RNA virus and is believed to have zoonotic origin.1 It was first identified in Wuhan, China, in December 2019 where it spread over a wide geographic area until it reached the status of a pandemic in 2020.2
The mortality rate for COVID-19 infection is approximately 5% but greatly increases with age (up to 15% in patients > 80 years of age) and the presence of comorbidities.3 The virus itself has cytopathic effects, commonly damaging the alveolar tissue in the lungs but can directly affect other organs.4 Additionally, clinical deterioration has been attributed to the cytokine storm, causing an acute inflammatory response due to immune dysregulation which consequently leads to organ failure and death.5
As more COVID-19 variants were identified, it became apparent that the virus was becoming more easily transmissible with less clinically apparent and less specific signs and symptoms, i.e. asymptomatic carriers causing accelerated dissemination as with the latest omicron strain.6 The more infectious variants which are now dominant are believed to have stemmed from the delta variant.7
Since the virus's main point of entry is via the respiratory tract, patients first present with non-specific viral lower respiratory tract symptoms - non-productive dry cough, dyspnoea, and persistent fever.8 As the disease progresses, patients develop respiratory distress signs and symptoms, coupled with low oxygen saturation (< 92%).9
Computed tomography (CT) scan is a highly sensitive modality for diagnosing COVID-19 pneumonia as changes may be seen early during the disease.10 Testing for the presence of viral particles on reverse transcriptase polymerase chain reaction (RT-PCR) can be used for screening purposes and as an adjunct to the CT scan to confirm that coronavirus is the pathogen involved.
Data surrounding patients with incidental COVID-19 in the surgical setting is scarce. Symptoms and signs of COVID-19 remain the same in both surgical and non-surgical patients. Screening of the asymptomatic cases was required. Some studies were conducted to evaluate the performance of CT scans in asymptomatic cases. Approximately 50% of patients enrolled showed the typical ground-glass appearance while the other 20% had atypical features.11 The remaining 30% did not have any features of infection.12
Other studies concluded that using a combination of symptom questionnaires and COVID-19 RT-PCR test are the two most important screening modalities when it comes to preoperative workup and diagnosing patients with incidental COVID-19.13 Although chest CT has high sensitivity (67-99%), it has relatively low specificity for COVID-19 (as low as 25%) and therefore does not add substantial value to the screening process, especially when used as a sole screening tool.14
Overall, patients who undergo surgical interventions during the incubation period of the virus and have symptoms, are more likely to require ICU admission than patients who tested negative for COVID-19.15,16 Longer procedures, older age, and the presence of comorbidities all play a pivotal role in the patient's postoperative outcome.1516 We postulated that patients who were diagnosed with incidental COVID-19, and underwent surgery, did not have a worse outcome due to the COVID-19 virus compared to their counterparts who did not have the virus.
Methods
This retrospective study included surgical patients (COVID-19 incidentals and COVID-19 negatives) who were admitted to a tertiary surgical intensive care unit at Tygerberg Academic Hospital, between 1 May 2020 and 31 December 2021. During the specified period of this study, it was hospital policy that all patients requiring intensive care admission be tested for COVID-19 to cohort the patients that have COVID-19 in the same ICU space and prevent further spread (Figure 1). The patients did not have a COVID-19 RT-PCR test done preoperatively and were screened for COVID-19 symptoms before admission to the hospital. The following characteristics were used as the inclusion criteria: adult patients (18 years or older), admitted to the surgical ICU, who underwent a surgical procedure and/or further management of surgical pathology. Descriptive statistics were calculated for all variables of interest. Means and standard deviations (or medians and interquartile ranges, where appropriate) were calculated for all variables concerned. Continuous variables were tested for normal distributions using the Kolmogorov-Smirnov test, and groups were compared using suitable parametric or non-parametric tests, as appropriate. The chi-squaredtest was used to compare categorical variables between groups. Additionally, group comparability was achieved through the utilisation of a regression model (binary). By employing this approach, we attempted to effectively assess the impact of various variables on the outcome variable. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS v. 28. Confounders were matched and two groups were compared after matching. Multivariate and univariate analysis were used.
Results
The sample consisted of 578 patients. The mean age was 43.9 years (SD = 16.7 years; range = 13.0-82.0 years) and 181 (31.3%) were female. One hundred and fifty-six patients (27.0%) had hypertension, 37 (6.4%) had asthma and/or COPD, 32 (5.5%) had cardiac disease, 30 (5.2%) had diabetes mellitus, 28 (4.8%) had dyslipidaemia, 16 (2.8%) had peripheral vascular disease, 8 (1.4%) had previously had a cerebrovascular accident, and 5 (0.9%) had tuberculosis infection at the time of ICU admission (Table I). Forty-one patients (7.1%) were known to be HIV-positive, 222 (38.4%) were known to be HIV-negative, and the remaining 315 (54.5%) had unknown HIV statuses. Of the 41 known HIV-positive patients, 25 (61.0%) were on antiretroviral therapy. The median CD4 count was 240.0 cells/μL (IQR = 118.5-430.0 cells/μL; Range = 0.0-1012.0 cells/μL).
Two hundred and thirty-four patients (40.5%) were admitted by the department of abdominal surgery, 229 (39.6%) by the department of trauma surgery, 57 (9.9%) by the department of vascular surgery, 43 (7.4%) by the department of orthopaedic surgery, 10 (1.7%) by the department of breast and endocrine surgery, and 5 (0.9%) by the department of neurosurgery. The most common diagnoses were acute appendicitis with generalised peritonism (2.8%), perforated peptic ulcer (2.4%), necrotising fasciitis (2.2%), malignant neoplasm of the colon (2.2%) and acute abdomen with unspecified aetiology (1.9%%). Four hundred and ninety-eight patients (86.2%) underwent surgery, while 80 (13.8%) did not. Forty-one patients (7.1%) were found to have incidental COVID-19 infection, while 537 (92.9%) tested negative for COVID-19 infection. The mean age of patients with COVID-19 infection (35.5 years) was significantly lower than that of patients without (44.6 years; p = 0.001); however, patients with and without COVID-19 infection were similar in sex, prevalence of all comorbidities, and diagnoses.
The median APACHE II score was 8.0 (IQR = 4.0-14.0; range = 0.0-60.0). Patients with hypertension had significantly higher APACHE II scores (median = 10.0; IQR = 5.8-16.3) than those without (median = 7.0; IQR = 3.0-13.0; p < 0.001). Patients with cardiac disease had significantly higher APACHE II scores (median = 10.5; IQR = 7.0-18.3) than those without (median = 8.0; IQR = 4.0-14.0; p = 0.03). The APACHE II scores of patients with COVID-19 infection (median = 8.0; IQR = 3.0-12.0) and those without (median = 8.0; IQR = 4.0-14.0) were similar (p = 0.71).
One hundred and twenty patients (20.8%) required transfusion of blood products in the ICU. Logistic regression showed that no demographic factors or comorbidities were significant predictors of patients requiring blood transfusion. The rates of blood transfusion in patients with COVID-19 infection (24.4%) and those without (20.5%) were similar (p = 0.55) (Table II).
In total, two hundred and twenty patients (38.1%) required inotropic support. Logistic regression showed that older patients were significantly more likely to require inotropic support (p = 0.05), however, no other demographic factors or comorbidities were significant predictors of patients requiring inotropic support. The rates of inotropic support in patients with COVID-19 infection (41.5%) and those without (37.8%) were similar (p = 0.64) (Table II).
The median duration of inotropic support for the entire study population was 2.0 days (IQR = 1.0-3.0 days; range = 1.0-10.0 days). Spline regression showed that no demographic factors or comorbidities were significant predictors of the duration of inotropic support. The duration of inotropic support in patients with COVID-19 infection (median = 2.0 days; IQR = 1.0-4.0 days) and those without (median = 2.0 days; IQR = 1.0-3.0 days) were similar (p = 0.11).
Thirty-two patients (5.5%) did not require any ventilatory support, while 167 (28.9%) required supplemental oxygen via facemask, 358 (61.9%) required endotracheal intubation and 21 (3.6%) required a tracheostomy. There were no significant associations between any demographic factors or comorbidities and the extent of ventilatory support needed. There were no significant associations between COVID-19 infection and the extent of ventilatory support needed (p = 0.13) (Table II).
The median time on ventilatory support was 2.0 days (IQR = 1.0-5.0 days; range = 1.0-38.0 days). Spline regression showed that no demographic factors or comorbidities were significant predictors of the duration of ventilatory support. The duration of ventilatory support in patients with COVID-19 infection (median = 2.0 days; IQR = 1.0-5.0 days) and those without (median = 3.0 days; IQR = 1.0-5.0 days) were similar (p = 0.73).
One hundred and eighty-two patients (31.5%) developed acute renal failure in the ICU, with 83 (45.6%) of these having AKIN stage 1 renal failure, 40 (22.0%) stage 2, and 59 (32.4%) stage 3. Logistic regression showed that older patients were significantly more likely to develop acute renal failure (p = 0.03); however, no other demographic factors or comorbidities were significant predictors of acute renal failure. The incidences of acute renal failure in patients with COVID-19 infection (26.8%) and those without (31.8%) were similar (p = 0.5). Thirteen patients (2.3%) received dialysis during their ICU stay. Logistic regression showed that no demographic factors or comorbidities were significant predictors of patients requiring dialysis. The rates of dialysis in patients with COVID-19 infection (2.4%) and those without (2.2%) were similar (p = 0.93) (Table II).
Of the 554 patients whose nutritional statuses were documented, 394 (71.1%) received oral feeds in the ICU, while 72 (13.0%) required enteral feeds, 26 (4.7%) required parenteral nutrition, and 62 (11.2%) died before receiving any nutrition in the ICU. There were no significant associations between any demographic factors or comorbidities and the extent of nutritional support needed. There were no significant associations between COVID-19 infection and the extent of nutritional support needed (p = 0.19).
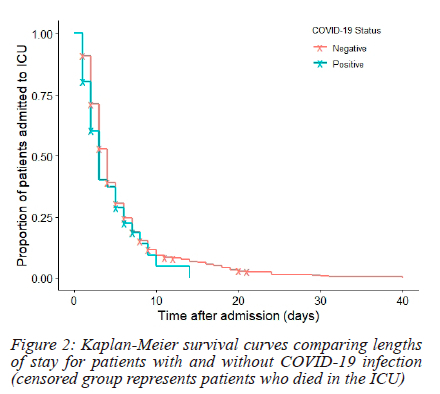
The median length of ICU stay was 3.0 days (IQR = 2.0-5.0 days; range = 1.0-40.0 days). Spline regression showed that no demographic factors or comorbidities were significant predictors of length of stay. The duration of ICU stay in patients with COVID-19 infection (median = 3.0 days; IQR = 2.0-5.0 days) and those without (median = 3.0 days; IQR = 2.0-5.0 days) were similar (p = 0.10).
Survival distributions of patients with and without COVID-19 infection were similar (p = 0.21; refer to Figure 2).
Four hundred and sixty-eight patients (81.0%) had uncomplicated ICU admissions, while 30 (5.2%) had admissions associated with complications, and a further 80 (13.8%) died in the ICU. Logistic regression showed that no demographic factors or comorbidities were significant predictors of death or complications. The rates of complications in patients with COVID-19 infection (7.3%) and those without (5.0%) were similar (p = 0.64). Grades of complications, as measured using the Clavien-Dindo classification were also similar between patients with and without COVID-19 infection (p = 0.19). The mortality rates of patients with COVID-19 infection (17.1%) and those without (13.6%) were similar (p = 0.53).
Post-hoc power analysis (α = 0.05) showed that the powers to detect significant differences in rates of blood transfusions (0.10), inotropic support (0.07), dialysis (0.04), complications (0.13) and mortality (0.11), and durations of inotropic support (0.20), ventilation (0.15), and ICU admission (0.54) were inadequate (< 0.8), implying that larger sample size was needed to reliably detect differences or conclude similarity in these variables.
Discussion
During the early stages of the pandemic, the morbidity and mortality rate for COVID-19 was increased in surgical patients, owing most likely to a lack of knowledge of the virus itself and a lack of treatment modalities.17 The rates of patients undergoing emergency surgery and elective surgery were higher.17 Patients who had nosocomial COVID-19 infection postoperatively had increased rates of morbidity and mortality.14,18 This was also exacerbated by longer hospital stays and delays in the diagnosis of various gastrointestinal conditions.18 This could also be attributed to delays in screening services and outpatient routine follow-up.18 However, as our understanding of the virus and treatment strategies improved, our morbidity and mortality rates in patients with incidental COVID-19 began to approach those patients without COVID-19.19
The COVID-19 pandemic has had a significant impact on how surgery was practised and how we managed those patients postoperatively.20 However, as the pandemic progressed, our study and various international studies failed to show a significant difference in the outcomes in patients with asymptomatic COVID-19 and patients who were COVID-19 positive.19,21 This was evident during both surge and non-surge periods during the pandemic.20,22 This is consistent with international literature both in Europe and America.19,20 Our median APACHE scores were similar in both the incidental COVID-19 group and those without COVID-19 when accounting for variables within the group. The findings align with the study of Mehryar et al.23 This is indicative of the utility of APACHE II scores in predicting disease severity and mortality.23 The incidence of surgical site sepsis in patients with incidental COVID-19 and COVID-19 negative were similar. This is consistent in the literature as seen in the study of Smith et al.24
The duration and need for ventilatory support were consistent in both incidental COVID-19 and COVID-19 negative groups. This is consistent with international literature as seen by Zeng et al.25 The presence of incidental COVID-19 in our cohort, did not significantly alter the need for ICU-specific interventions such as blood transfusions, inotropic support, or ventilatory assistance. The study of Doglietto et al. compared patients with incidental COVID-19 to patients who were COVID-19 negative and found that hospital length of stay, ventilator days, and post-acute care facility length of stay were longer in direct contradiction to our study.26 Furthermore, the study of Yeates et al. showed a fourfold mortality rate, longer length of stay, and ICU length of stay which were mostly related to pulmonary and thrombotic complications in the incidental COVID-19 group.27 However, the overall trend in these studies showed that those with asymptomatic COVID-19 fared worse about ICU length of stay and ventilator associated days.23,27,28 This was inconsistent with our study and later available literature.18
Testing patients preoperatively may incur unnecessary hospital expenditure and may be labour intensive.29 The most recent literature would suggest that having asymptomatic COVID-19 does not affect morbidity and mortality.18,30 Thus, we can infer that testing asymptomatic COVID-19 may offer little benefit outside of a research setting.19
In addition to the findings above, the cost of hospitalisation of patients with asymptomatic COVID-19 patients equated to €7 185.8 per hospital admission.31 This equated to an approximate cost of €105 933 677.6 in totality to the country of Spain in 2020.31 It went on further to say that the evaluation of costs showed that patients admitted with asymptomatic COVID-19 had higher mean costs per patient (+26.4%) than those found in a previous study of hospitalised patients with symptomatic COVID-19. Seguí et al. showed that the benefit-cost ratio excludes the health budget of a mass screening intervention in the asymptomatic general population as it approaches 0.45.32 The social return on the investment of one euro is therefore only 45 cents in the asymptomatic population.32 Given our resource deprived settings, South Africa can ill afford routine COVID-19 testing for asymptomatic patients. There is, however, a paucity of data for the South African setting about asymptomatic COVID-19 hospitalisations. This could be a source for further studies in the future.
In Massey et al. orthopaedic patients treated at a level 1 trauma centre showed no increase in mortality in patients with asymptomatic COVID-19.33 The median age, cohort and demographic were similar to our cohort of patients. There was no difference in length of stay and mortality but the ICU stay was slightly increased.33
There are multiple limitations to our study. This was a retrospective study; therefore, we could only extract data from the available datasets. This was a single-centre study, which limits the generalisation of the findings in our study. Lastly, since we have no gold standard test to identify true positives, we must consider that false positive PCR test results might exist and negatively affect statistical inferences within both groups.
Conclusion
This study demonstrates that surgery among asymptomatic PCR-positive patients was not associated with increased mortality or morbidity in the SICU. This also adds a valuable contribution to the growing body of literature regarding COVID-19 infections. Further prospective and multi-centred studies are required to provide more robust results.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No external or internal funding was utilised to generate this work.
Ethical approval
Ethical approval was obtained from the Stellenbosch University Human Research Ethics Committee (HREC S23/10/227).
ORCID
M Parker https://orcid.org/0009-0009-6231-2089
I Mia https://orcid.org/0000-0002-2446-960X
N Ahmed https://orcid.org/0000-0002-3916-5027
V van der Westhuizen https://orcid.org/0009-0009-2900-5896
A Diayar https://orcid.org/0009-0008-1683-7960
J Buitendag https://orcid.org/0000-0001-7169-129X
REFERENCES
1. Hasöksüz M, Kiliç S, Saraç F. Coronaviruses and Sars-Cov-2. Turk J Med Sci. 2020;50(SI-1):549-56. https://doi.org/10.3906/sag-2004-127. [ Links ]
2. Lin YF, Duan Q, Zhou Y, et al. Spread and impact of COVID-19 in China: a systematic review and synthesis of predictions from transmission-dynamic models. Front Med (Lausanne). 2020;7:1-11. https://doi.org/10.3389/fmed.2020.00321. [ Links ]
3. Djaharuddin I, Munawwarah S, Nurulita A, et al. Comorbidities and mortality in COVID-19 patients. Gac Sanit. 2021;35:S530-S532. https://doi.org/10.1016/j.gaceta.2021.10.085. [ Links ]
4. Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611 583 subjects. J Am Med Dir Assoc. 2020;21(7):915-8. https://doi.org/10.1016/j.jamda.2020.05.045. [ Links ]
5. Nazerian Y, Ghasemi M, Yassaghi Y, Nazerian A, Hashemi SM. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int Immunopharmacol. 2022;113. https://doi.org/10.1016/j.intimp.2022.109428. [ Links ]
6. Islam S, Islam T, Islam MR. New coronavirus variants are creating more challenges to global healthcare system: A brief report on the current knowledge. Clin Pathol. 2022;15. https://doi.org/10.1177/2632010X221075584. [ Links ]
7. Andre M, Lau LS, Pokharel MD, et al. From alpha to omicron: how different variants of concern of the SARS-Coronavirus-2 impacted the world. Biology (Basel). 2023;12(9). https://doi.org/10.3390/biology12091267. [ Links ]
8. Parasher A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312-20. https://doi.org/10.1136/postgradmedj-2020-138577. [ Links ]
9. Baj J, Karakula-Juchnowicz H, Teresinski G, et al. COVID-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J Clin Med. 2020;9(6):1-22. https://doi.org/10.3390/jcm9061753. [ Links ]
10. Garg M, Prabhakar N, Bhalla A, et al. Computed tomography chest in COVID-19: When & why? Indian J Med Res. 2021;153(1):86-92. https://doi.org/10.4103/ijmr.IJMR_3669_20. [ Links ]
11. Huybens EM, Bus MPA, Massaad RA, et al. What is the preferred screening tool for COVID-19 in asymptomatic patients undergoing a surgical or diagnostic procedure? World J Surg. 2020;44(10):3199-206. https://doi.org/10.1007/s00268-020-05722-9. [ Links ]
12. Ord AA, Zamparini J, Lorentz L, Ranchod A, Moodley H. A study of the chest imaging findings of adult patients with COVID-19 on admission to a tertiary hospital in Johannesburg, South Africa. S Afr J Infect Dis. 2022;37(1). https://doi.org/10.4102/sajid.v37i1.449. [ Links ]
13. Singhal R, Ludwig C, Rudge G, et al. 30-Day morbidity and mortality of bariatric surgery during the COVID-19 pandemic: a multinational cohort study of 7704 patients from 42 countries. Obes Surg. 2021;31(10):4272-88. https://doi.org/10.1007/s11695-021-05493-9. [ Links ]
14. Aziz S, Arabi YM, Alhazzani W, et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46(7):1303-25. https://doi.org/10.1007/s00134-020-06092-5. [ Links ]
15. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21. https://doi.org/10.1016/j.eclinm.2020.100331. [ Links ]
16. Aziz H, Filkins A, Kwon YK. Review of COVID-19 outcomes in surgical patients. American Surgeon. 2020;86(7):741-745. https://doi.org/10.1177/0003134820934395. [ Links ]
17. Yilmaz S, Sapci I, Jia X, et al. Risk factors associated with postoperative mortality among COVID-19 positive patients: Results of 3027 operations and procedures. Ann Surg. 2022;276(6):969-974. https://doi.org/10.1097/SLA.0000000000005722. [ Links ]
18. Gupta R, Gupta J, Ammar H. Impact of COVID-19 on the outcomes of gastrointestinal surgery. Clin J Gastroenterol. 2021;14(4):932-946. https://doi.org/10.1007/s12328-021-01424-4. [ Links ]
19. Aydın O, Ergen P, Vahaboglu H. Safety of surgery among asymptomatic SARS-CoV-2 PCR-positive patients: A single-center retrospective cohort study. World J Surg. 2023;47(3):573-577. https://doi.org/10.1007/s00268-023-06891-z. [ Links ]
20. Yoshida T, Chude-Sokei R, Araji T, Adra S. Impact of COVID-19 pandemic surgeon surgical outcomes: A retrospective study. Am Surg. 2024;90(6). https://doi.org/10.1177/00031348241227213. [ Links ]
21. Cai M, Wang G, Zhang L, et al. Performing abdominal surgery during the COVID-19 epidemic in Wuhan, China: a single-centred, retrospective, observational study. Br J Surg. 2020;107(7). https://doi.org/10.1002/bjs.11643. [ Links ]
22. Caballero-Milán M, Colomina MJ, Marin-Carcey LA, et al. Impact of the SARS-CoV-2 (COVID-19) pandemic on the morbidity and mortality of high-risk patients undergoing surgery: a non-inferiority retrospective observational study. BMC Anesthesiol. 2021;21(1). https://doi.org/10.1186/s12871-021-01495-3. [ Links ]
23. Mehryar HR, Yarahmadi P, Anzali BC. Mortality predictive value of APACHE II scores in COVID-19 patients in the intensive care unit: a cross-sectional study. Ann Med Surg. 2023;85(6):2464-8. https://doi.org/10.1097/MS9.0000000000000641. [ Links ]
24. Smith BB, Bosch W, O'Horo JC, et al. Surgical site infections during the COVID-19 era: a retrospective, multicentre analysis. Am J Infect Control. 2023;51(6):607-11. https://doi.org/10.1016/j.ajic.2022.09.022. [ Links ]
25. Zeng H, Ma Y, Zhou Z, et al. Spectrum and clinical characteristics of symptomatic and asymptomatic coronavirus disease 2019 (COVID-19) with and without pneumonia. Front Med (Lausanne). 2021;8. https://doi.org/10.3389/fmed.2021.645651. [ Links ]
26. Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691-702. https://doi.org/10.1001/jamasurg.2020.2713. [ Links ]
27. Yeates EO, Grigorian A, Schellenberg M, et al. COVID-19 in trauma: a propensity-matched analysis of COVID and non-COVID trauma patients. Eur J Trauma Emerg Surg. 2021;47(5):1335-42. https://doi.org/10.1007/s00068-021-01699-9. [ Links ]
28. Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020;44(8):2477-81. https://doi.org/10.1007/s00268-020-05575-2. [ Links ]
29. Evans S, Naylor NR, Fowler T, Hopkins S, Robotham J. The effectiveness and efficiency of asymptomatic SARS-CoV-2 testing strategies for patients and healthcare workers within acute NHS hospitals during an omicron-like period. BMC Infect Dis. 2024;24(1). https://doi.org/10.1186/s12879-023-08948-9. [ Links ]
30. Cano-Valderrama O, Morales X, Ferrigni CJ, et al. Acute care surgery during the COVID-19 pandemic in Spain: Changes in volume, causes and complications. A multicentre retrospective cohort study. Int J Surg. 2020;80. https://doi.org/10.1016/j.ijsu.2020.07.002. [ Links ]
31. Álvarez-del Río B, Sánchez-de Prada L, Álvaro-Meca A, et al. Prevalence and cost of hospitalised patients with asymptomatic COVID-19 in 2020 in Spain. Front Public Health. 2023;11. https://doi.org/10.3389/fpubh.2023.1229561. [ Links ]
32. Seguí FL, Cuxart OE, Mitjà I Villar O, et al. Public health article analysis of the COVID-19 asymptomatic mass testing strategy in the north metropolitan area of Barcelona. Int J Environ Res. 2021;18:7028. https://doi.org/10.3390/ijerph18137028. [ Links ]
33. Massey PA, Andre LK, Kautz SM, et al. Low mortality of orthopaedic trauma patients with asymptomatic COVID-19: A level 1 trauma centre pandemic experience. Ochsner Journal. 2022;22(3). https://doi.org/10.31486/toj.21.0117. [ Links ]
 Correspondence:
Correspondence:
email: muzaffarparker@gmail.com













