Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489Print version ISSN 0038-2353
S. Afr. j. sci. vol.106 n.3-4 Pretoria Mar./Apr. 2010
RESEARCH ARTICLE
The production and use of citric acid for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine, South Africa
Peter J. WilliamsI; Thomas E. CloeteII
IDepartment of Chemical Engineering, Faculty of Engineering, Built Environment and IT, University of Pretoria, South Africa
IIScience Faculty, Stellenbosch University, South Africa
ABSTRACT
The depletion of the richer iron ore worldwide has made it necessary to process lower quality iron ore. Certain substances, such as potassium, contained within the lower quality iron ore, have a detrimental effect on the smelting process during steel manufacturing. Therefore, international steel-making companies charge penalties when purchasing iron ore concentrates containing high concentrations of potassium. To date, lower quality iron ore has been blended with high quality iron ore in an attempt to alleviate the potassium concentrations in the export iron ore; however, the ratio of low quality iron ore to high quality iron ore is increasing, thereby becoming an escalating problem within the economic functioning of the Sishen Iron Ore Mine. It has, therefore, become necessary to develop an economically viable and environmentally friendly process to reduce the high potassium concentrations contained in the iron ore concentrate of the Sishen Iron Ore Mine. In this study, we compared solid substrate and submerged fermentation using Aspergillus niger for the production of citric acid, which is used for the chemical leaching of potassium from the iron ore concentrate. It was found that submerged fermentation proved to be more economical and efficient, producing a maximum citric acid concentration of 102.3 g/L in 96 h of fermentation. 'Heap leaching' simulation experiments were found to be uneconomical, due to the required addition of fungal growth medium every 5 days as a result of growth factor depletion within this time; however, this process removed 17.65% of the potassium from the iron ore concentrate. By contrast, chemical leaching of potassium from the iron ore concentrate proved to be most efficient when using a 1 mol citric acid leaching solution at 60 ºC, removing 23.53% of the potassium contained within the iron ore concentrate. Therefore, the most economical and efficient process for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine involved a two-stage process whereby citric acid was produced by A. niger, followed by the chemical leaching of the potassium from the iron ore concentrate using a 1 mol citric acid leaching solution at 60 ºC.
Keywords: chemical leaching; heap leaching; potassium; solid substrate citric acid fermentation; submerged citric acid fermentation
INTRODUCTION
The depletion of the richer iron ore deposits worldwide, as a result of advancing global technologies and civilisation, has necessitated the processing of lower quality iron ore.1 The potassium contained within lower quality iron ore has a detrimental effect on the smelting process during steel-making in blast furnaces.2 Potassium is deposited on the surface of the coke, where it acts as a catalyst in the gasification of carbon in the presence of carbon dioxide.2 The presence of potassium in the coke leads to the formation of potassium silicate and potash feldspar, which leads to an increase in the coke volume and its subsequent fracture.2 In addition, potassium penetrates the monolithic aluminosilicate lining of the furnace, resulting in the formation of silicide or leucite, and the subsequent rearrangement of the crystalline lattice of the refractories.2 This results in the creation of stresses that cause cracks to form in the refractory lining, leading to its subsequent destruction.2 Therefore, as a result of the negative financial impact due to the destruction of the refractory lining, steel-making companies charge penalties when purchasing iron ore concentrates that contain high concentrations of potassium. The limits on alkali concentrations range from 0.25% mass in Japan to 0.55% mass in Switzerland.2
In the past, lower quality iron ore was blended with high quality ore to 'dilute' the potassium concentration in the final iron ore product, which is exported to international steel-making plants. Similar practices have been reported from other parts of the world, such as the Hamersley Province in Australia, where low-phosphorous ore (0.05%) is blended with high-phosphorous ore (0.1%), the former being the major component of the blend.3 To date, the blending of different quality iron ores has minimised the penalties charged by steel-making companies. However, the ratio of low quality iron ore to high quality iron ore is increasing; this is becoming an escalating problem within the economic functioning of the Sishen Iron Ore Mine. It has, therefore, become important to develop an economically viable and environmentally friendly process to reduce the high potassium concentration contained in the iron ore concentrate, to improve the quality of ore that is being exported to international steel-making companies.
During a previous study (Table 1), in which a range of inorganic and organic acids were tested for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine, it was discovered that citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid) proved to be the best leaching agent for the removal of potassium, without a major reduction in the iron content (0.46%) of the ore. Citric acid is an intermediate in the tricarboxylic acid (TCA) cycle, which is widely used in food, beverage, pharmaceutical and cosmetic industries, but also has other applications in textile, electroplating and bioremediation industries.4,5,6 The most popular microorganism for the large-scale production of citric acid is the white-rot fungus Aspergillus niger, due to its high citric acid productivity at low pH, without the secretion of toxic metabolites.7 Citric acid production by A. niger involves two main metabolic pathways, namely, (1) the catabolic pathway of hexoses to pyruvate and acetyl-coenzyme A by glycolysis and (2) citric acid formation by the TCA cycle.8 During cell propagation and maintenance, complete oxidation of glucose leads to the production of adenosine triphosphate (ATP), carbon dioxide and water.9 Depending on the growth conditions and concentrations of end products, A. niger is able to control when to stop the full respiration process during the TCA cycle, resulting in the production of citric acid.9
Citric acid contains three carboxyl groups, which tend to donate protons (H+), resulting in negatively charged carboxyl groups that are capable of forming stable complexes with several cations.10 Therefore, it is possible that these negatively charged carboxyl groups might form stable complexes with the positively charged potassium cations present, resulting in their removal from the iron ore concentrate. Therefore, the objective of this study was to develop a 'bioleaching' process, whereby citric acid is produced and then used to remove the potassium from the iron ore concentrate of the Sishen Iron Ore Mine. In addition, a 'heap leaching' process was investigated, whereby A. niger was directly applied to the iron ore concentrate of the Sishen Iron Ore Mine.
MATERIALS AND METHODS
Microorganism and preparation of inoculum
A freeze-dried sample of A. niger NRRL 567 was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and stored at 4 ºC. A. niger spores were produced on potato dextrose agar (PDA) (Merck Laboratories, Darmstadt, Germany) at 30 ºC and were subcultured at biweekly intervals. Spores were harvested after 7 days of incubation by adding 10 mL of 0.1% Tween 80 (Merck) solution to each plate. Spore inoculates of 1.0 x 107 spores/mL were prepared using a haemocytometer.
Solid substrate
Sphagnum peat moss (Schultz Company, Mississauga, Canada) supplemented with glucose was used to simulate a sugar-rich by-product. The peat moss was sterilised in an autoclave at 121 ºC for 15 min. The peat moss was wetted to moisture content of 80% wet weight with deionised water, supplemented with various salts and glucose.11
Basal salt solution for solid substrate fermentation
The basal salt solution used to wet the dried peat moss and to provide basic nutrients to the fungal culture contained the following: 3.84 g/L ammonium sulphate, 10.98 g/L potassium phosphate, 1.01 g/L sodium chloride, 1.01 g/L magnesium sulphate and 1.01 g/L ferrous sulphate.7 After addition to the peat moss, the solution gave the following salt concentration in terms of kilograms of dried peat moss (DPM): 15.36 g ammonium sulphate, 43.92 g potassium phosphate, 4.04 g sodium chloride, 4.04 g magnesium sulphate and 4.04 g ferrous sulphate.
Fermentation medium for submerged fermentation
The fermentation medium for the submerged fermentation was prepared using distilled water and contained the following: 150 g/L D-glucose, 2.5 g/L ammonium sulphate, 0.5 g/L magnesium sulphate, 2 g/L potassium phosphate, 0.1 x 10-3 g/L ferric sulphate, 0.1 x 10-3 g/L zinc sulphate and 0.06 x 10-3 g/L copper sulphate.
Fermentation conditions for solid substrate fermentation
Solid substrate fermentation was conducted in 500-mL Erlenmeyer flasks holding 25 g of DPM wetted with 100 mL of the basal salt solution and 967.88 g/kg DPM glucose (Merck).7 The solid substrate was inoculated with 1 mL of A. niger NRRL 567 inoculum (1.0 x 107 spores/mL). The Erlenmeyer flasks and their contents were incubated at 30 ºC for 6 days. Fermentation procedures were conducted in duplicate.
Fermentation conditions for submerged fermentation
Submerged fermentation was conducted in 500-mL Erlenmeyer flasks. Briefly, 250 mL of fermentation medium was inoculated with 1 mL of A. niger NRRL 567 inoculum (1.0 x 107 spores/mL). The Erlenmeyer flasks and their contents were incubated at 30 ºC for 6 days. Again, fermentation procedures were conducted in duplicate.
Sample preparation
For each sampling procedure of the solid substrate fermentation, 5 g of a wet sample of peat moss was harvested from each flask and placed in 50 mL distilled water, followed by incubation in a shake incubator for 60 min at 150 revolutions per minute at 25 ºC. The supernatant was filtered through a 0.45-µm syringe filter (Millipore, Billerica, MA, USA), followed by analyses for pH and citric acid quantification. Sampling of the submerged fermentation entailed the aseptic extraction of 5 mL of the fermentation medium from each flask to be used for analyses. The extracted medium was filtered through a 0.45-µm syringe filter (Millipore), followed by analyses for pH and citric acid quantification, which were performed daily for 6 days.
Citric acid concentration analysis
Citric acid concentrations were determined by spectrophotometry at 420 nm after adding pyridine and acetic anhydride.12 In brief, 1 mL of the sample was added to a test tube containing 1.3 mL pyridine (Merck), followed by the addition of 5.7 mL acetic anhydride (Merck). The contents of the test tube were mixed manually by swirling and immediately placed in a constant-temperature (22 ºC) water bath. Colour development was allowed for 30 min, followed by reading the colour intensity at 420 nm with the blank set on 100% transmission. The citric acid concentration was determined by referring to a standard curve for citric acid concentration. Citric acid concentrations were expressed per kilogram of DPM for solid substrate fermentation and grams per litre for submerged fermentation.
Chemical leaching of iron ore concentrate with citric acid
The citric acid produced was used for chemical leaching of the iron ore concentrate. A 1 mol concentration (40 mL) of the produced citric acid was prepared and added to a 40 g sample of iron ore concentrate (-5+1 mm particle size) in a 250-mL Erlenmeyer flask. A 40 g sample of iron ore concentrate containing 40 mL of distilled water was used as a negative control. The flasks were incubated at 25 ºC for 5 days. In addition, the leaching procedure was conducted using different concentrations (0.25 mol, 0.5 mol, 0.75 mol and 1 mol) of citric acid at a leaching temperature of 60 ºC for 5 days. After the incubation, the leaching solutions were removed and the iron ore concentrate washed five times with distilled water. The treated iron ore concentrate samples were dried in an oven at 150 ºC for 24 h. The iron ore concentrate samples were stored in a dry environment for chemical analysis.
Heap leaching of iron ore concentrate using Aspergillus niger
A heap leaching simulation experiment was conducted in 500-mL Erlenmeyer flasks. Spore suspensions were prepared as described above in the preparation of the inoculum. The fermentation medium used during these experiments was the same as used for submerged citric acid fermentation. Iron ore concentrate (500 g) was placed in each Erlenmeyer flask and mixed with 50 mL of fermentation medium inoculated with 1 mL of the spore inoculum (1.0 x 107 spores/mL). The flasks and their contents were incubated at 30 ºC for a period of 40 days.
Chemical analysis of the treated iron ore concentrate
The chemical content of the treated iron ore concentrate was analysed by the Department of Geology, University of Pretoria, Pretoria, South Africa, using X-ray fluorescence (XRF) spectrometry of the major elements. The samples were ground to < 75 µm in a tungsten carbide milling vessel and roasted at 1000 ºC, before 1 g of sample and 9 g Li2B4O7 were fused into a glass bead. Major element analysis was executed on the fused bead using an ARL9400XP+ spectrometer. Another aliquot of the sample was pressed into a powder briquette for trace element analysis.
RESULTS AND DISCUSSION
Previous research has suggested that citric acid is excreted from A. niger cells in response to unfavourable intracellular conditions that lead to increased levels of tricarboxylic acids through anaplerotic pathways during growth in a high-glucose concentration environment.13 It is suggested that polyols, in particular glycerol, may play an important role as osmoregulators in A. niger cells, which explains their ability to grow in environments where high glucose concentrations prevail.14 Therefore, a high initial glucose concentration of 967.88 g/kg DPM and 150 g/L was used for solid substrate and submerged fermentation respectively, to ensure that citric acid was excreted from the A. niger mycelia.
Solid substrate and submerged citric acid production by A. niger NRRL 567 at 30 ºC is illustrated in Figures 1 and 2, respectively. Citric acid excretion commenced after approximately 24 h during solid substrate fermentation, compared to 48 h during submerged fermentation. This difference may be attributed to abnormal spore germination in the form of bulbous cells during the early stages of growth, followed by a sudden change in morphology to highly branched filamentous hyphae, which are responsible for the citric acid overflow.15 During the germination of the fungal spores, the pentose phosphate pathway is predominant, followed by a switch to glycolysis before the onset of citric acid excretion.16,17
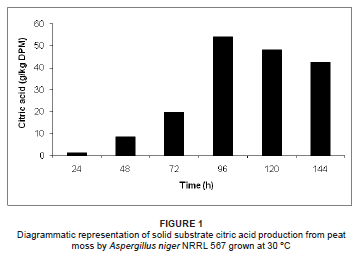
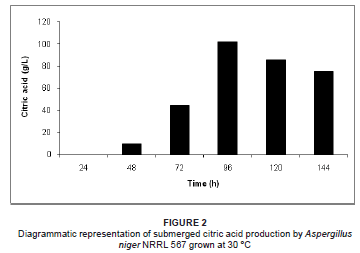
An increase in citric acid productivity was observed until maximisation at approximately 96 h (54.2 g/kg DPM and 102.3 g/L respectively for solid substrate and submerged fermentation) (Figures 1 and 2). Similar observations have been recorded, where little or no citric acid could be detected in the medium during the first 24 h of fermentation, while a relatively slow excretion rate was observed during the second day of fermentation, followed by a sudden increase in citric acid productivity thereafter.18,19,20,21 During the productive phase of citric acid accumulation, the direct conversion of hexoses to pyruvate via glycolysis becomes predominant, starting after approximately 24 h and accelerating after 40 h - 50 h of growth in a batch system.16 However, the mechanism causing the shift of glucose degradation from the pentose phosphate pathway to glycolysis is not clearly understood.13
To attain increased citric acid yields, nitrogen in the medium must be limiting.22 Citric acid production is only established once the nitrogen in the medium is depleted.21 The bulk of the ammonium is removed from the medium between 20 h and 25 h, followed by the release of protons into the fermentation medium.21 In the early stage of citric acid production (< 24 h), it seems that a chain of events is established where fungal growth leads to ammonium uptake, which leads to proton release.21 It is suggested that a nitrogen compound must be produced and excreted by the mycelium, as the increase in biomass cannot be due to an accumulation of ammonia in the biomass of the fungus.21 It was subsequently reported that the nitrogen compound in question was in fact glucosamine, as identified by high-performance liquid chromatography.21 Following the detection of glucosamine, the formation, release and fate of the aminated compound throughout the fermentation process was investigated.21 It was found that the highest detected concentration of glucosamine in the medium was attained when using optimal concentrations of glucose and ammonium for citric acid production. Glucosamine excretion started at approximately 15 h of fermentation, followed by the start of citric acid production at about 24 h.21 It appears that the sudden increase in citric acid production is preceded by the sudden increase in glucosamine excretion into the medium by A. niger.21 Glucosamine appeared to be stable in the medium until approximately 85 h, whereafter it was reduced and subsequently depleted at 126 h of fermentation.21 This pattern correlates with the citric acid production peak of 54.2 g/kg DPM at 96 h for solid substrate fermentation and 102.3 g/L for submerged fermentation, followed by the decline of citric acid production thereafter (Figures 1 and 2). Therefore, when comparing the fate of glucosamine and citric acid production during the fermentation process, it appears that glucosamine plays a direct role in citric acid production. Although the direct role of glucosamine during fermentation is unclear, it is suggested that it acts as a storage compound and is utilised by the fungus during the course of fermentation.21
The initial pH decreased proportionally with the increase in citric acid concentration during both solid substrate and submerged fermentation. In both fermentation processes, the pH decreased during the first 96 h of fermentation as a result of citric acid production during this period. The maximum decreases in pH, 1.34 and 2.17 during solid substrate and submerged fermentation, respectively, were reached after 96 h, which correlates with the maximum citric acid concentrations achieved at this point during fermentation. In both solid substrate and submerged fermentation, the pH increased after 96 h due to the decreased citric acid concentrations in the fermentation media. It is evident that the evolution of the pH of the peat moss occurred in parallel with the citric acid concentration during both fermentation processes. Therefore, the pH evolution may be used as an indirect indicator of citric acid production during the fermentation process.
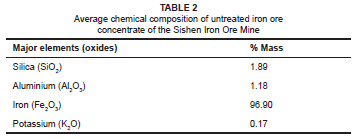
The chemical composition of the untreated iron ore concentrate of the Sishen Iron Ore Mine is given in Table 2. XRF spectrometry of the iron ore concentrate revealed a potassium concentration of 0.17% mass and an iron concentration of 96.9% mass.
The average percentage removal of the major elements from the iron ore concentrate of the Sishen Iron Ore Mine by chemical leaching for 5 days, using different concentrations of citric acid is given in Table 3. Based on the results listed in Table 3, it is evident that both the leaching temperature and the citric acid concentration play an important role during the chemical leaching of the potassium from the iron ore concentrate. Leaching at 30 ºC using a 1 mol citric acid leaching solution resulted in a 17.65% removal of potassium, while at 60 ºC, 23.53% of the potassium was removed (Table 3). Using a 1 mol citric acid leaching solution at 60 ºC also resulted in the increased removal of silica (15.87%), aluminium (18.64%) and iron (0.92%) compared to leaching at 30 ºC, where only 11.02% of aluminium, 0.46% of iron and no silica was removed from the iron ore concentrate (Table 3). Therefore, it is clear that chemical leaching of potassium from the iron ore concentrate occurred more efficiently at a higher temperature (60 ºC), which is most likely due to the fact that diffusion occurs more efficiently at higher temperatures.
Citric acid concentrations of 0.25 mol and 0.5 mol resulted in the removal of only 5.88 ± 5.88% of the potassium, while at citric acid concentrations of 0.75 mol and 1 mol, the potassium removal increased to 17.65 ± 5.88% and 23.53 ± 5.88%, respectively. Increasing the citric acid concentration beyond 1 mol, however, did not result in an increase in potassium removal from the iron ore concentrate. The reduced potassium removal using citric acid concentrations of 0.25 mol and 0.5 mol, however, could be as a result of data scatter due to sampling and assay variation, and may therefore be regarded as insignificant. The actual percentage decrease of potassium using citric acid concentrations of 0.75 mol and 1 mol may also be lower than recorded in Table 3, as a result of data scatter. When taking the data scatter into account, the actual decrease in potassium may be as low as 6.88% and 12.5%, using a citric acid concentration of 0.75 mol and 1 mol, respectively.
When comparing the use of different concentrations of citric acid for the chemical leaching of iron ore concentrate at 60 ºC, it was evident that the process occurs more efficiently at higher citric acid concentrations. The presence of a higher negatively charged carboxyl group concentration at higher citric acid concentrations leads to the formation of more stable complexes with the potassium cations present, resulting in a greater removal of potassium from the iron ore concentrate.
Other parameters that must be taken into account during chemical leaching of potassium from the iron ore concentrate are contact time and particle size of the iron ore concentrate, as well as the saturation state of the leaching system. During this investigation, it was found that the maximum potassium removal from the iron ore concentrate occurred after 5 days of chemical leaching, with no increase in potassium removal thereafter. Organic acids, such as citric acid, and their anions are able to affect mineral weathering by changing the dissolution rate of the mineral far from equilibrium by decreasing the solution pH or by forming stable complexes at the mineral surface.23 Previously, a surface complexation model has been proposed to describe mineral dissolution, where protonation of oxide minerals takes place on the mineral surface.24 Electrons are shifted towards the more electronegative oxygen, which consequently leads to the weakening of the bond that holds the metal in the mineral lattice.24 Therefore, particle size of the iron ore concentrate is suggested to be a limiting factor during this study. Decreasing the particle size would increase the relative surface area of the iron ore, resulting in a relative increase in surface complexation. Therefore, if treating iron ore of smaller particle size than -5+1 mm, relatively more potassium may be removed from the iron ore concentrate by surface complexation; however, the final iron ore concentrate product exported from the Sishen Iron Ore Mine has a particle size of -5+1 mm and, therefore, it was used during this study to investigate the removal of potassium from the iron ore concentrate using the smallest marketable particle size from the mine. Furthermore, from a previous study, in which the susceptibility of interlayer potassium in micas to exchange with sodium was investigated, it was found that, essentially, all potassium in muscovite, biotite, phlogopite and vermiculite was exchangeable when the concentration of the potassium in solution was kept low.25 Therefore, the limited amount of potassium leached from the iron ore concentrate may also be ascribed to the potassium saturation state of the leaching solution.
The heap leaching simulation experiments using A. niger for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine yielded various problems. A. niger was only able to proliferate on the surface of the iron ore concentrate layer in the flask, possibly due to the anaerobic conditions which may prevail within the iron ore concentrate layer. Therefore, oxygen would have to be introduced throughout the iron ore heap when using this type of technology. In addition, after a period of 5 days, the fungus started to sporulate, indicating the depletion of growth factors needed for fungal growth to occur. After the addition of more growth medium to the inoculated iron ore concentrate, fungal growth recommenced, followed by sporulation 5 days thereafter. Therefore, heap leaching technology would require the addition of fungal growth medium every 5 days, making this type of technology uneconomical due to the high costs of growth factors such as glucose.
During the 40-day heap leaching simulation, the pH of the leachate solution decreased from 4.00 to 3.42, indicating that a low concentration of citric acid was produced due to the limiting of the carbon source (glucose) in the system. XRF analysis revealed that 17.65% of the potassium was removed from the iron ore concentrate during the 40-day period. Therefore, compared to chemical leaching of the iron ore concentrate using a 1 mol concentration of citric acid produced by A. niger, heap leaching technology is less efficient as less potassium (17.65% vs 23.53%) is removed over a much longer contact time.
CONCLUSION
During solid substrate and submerged fermentation using A. niger, a high concentration of glucose was required to ensure that citric acid was excreted from the fungal mycelia. The maximum citric acid yield of 54.2 g/kg DPM by solid substrate fermentation and 102.3 g/L by submerged fermentation was attained after a period of 96 h in both processes. The fermentation media of both solid substrate and submerged fermentation contained similar constituents, with the only difference being that the submerged fermentation medium contained less of each constituent. In addition, solid substrate fermentation required peat moss as a solid substrate, which increases the costs incurred by using this fermentation process. Therefore, taking all the variable factors into account, it is suggested that submerged fermentation be used for citric acid production by A. niger, as this fermentation process was found to be more economical and produced more citric acid in the same time frame.
Chemical leaching of the iron ore concentrate proved to be more efficient than heap leaching, as more potassium was removed from the iron ore concentrate, as well as in a shorter time frame. In addition, using heap leaching technology proved to be uneconomical in this case, as growth medium would have to be added every 5 days to enhance fungal growth. It is suggested that a 1 mol citric acid leaching solution be used at 60 ºC for the chemical leaching process, as the most potassium is removed from the iron ore concentrate using these leaching conditions. Therefore, the most economical and efficient process for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine involves a two-stage process whereby citric acid is produced by A. niger, followed by the chemical leaching of the potassium from the iron ore concentrate, using a 1 mol citric acid leaching solution at 60 ºC.
ACKNOWLEDGEMENTS
The authors wish to thank Kumba Iron Ore, Ltd and the National Research Foundation of South Africa for the financial support received during this study.
REFERENCES
1. Jian N, Sharma DK. Biohydrometallurgy for non-sulphidic minerals - A review. Geomicrobiol J. 2004;21:135-144. [ Links ]
2. Yusfin YS, Chernousov PI, Garten V, Karpov YA, Petelin AL. The role of alkalis and conserving resources in blast-furnace smelting. Metallurgist. 1999;43:54-58. [ Links ]
3. Dukino RD, England BM, Kneeshaw M. Phosphorous distribution in BIF-derived iron ores of Hamersley Province, Western Australia. Trans Instn Min Metall (Sect B: Appl Earth Sci.) 2000;109:B168-B176. [ Links ]
4. Wang J, Liu P. Comparison of citric acid production by Aspergillus niger immobilized in gels and cryogels of polyacrylamide. J Ind Microbiol. 1996;16:351-353. [ Links ]
5. Tran CT, Sly LI, Mitchell DA. Selection of a strain of Aspergillus for the production of citric acid from pineapple waste in solid-state fermentation. W J Microbiol Biotechnol. 1998;14:399-404. [ Links ]
6. Ates S, Dingil N, Bayraktar E, Mehmetoglu U. Enhancement of citric acid production by immobilized and freely suspended Aspergillus niger using silicone oil. Process Biochem. 2002;38:433-436. [ Links ]
7. Kim J. Optimisation of citric acid production by Aspergillus niger NRRL 567 in various fermentation systems. Ph.D. thesis, McGill University, Ste-Anne-de-Bellevue, Canada; 2004. [ Links ]
8. Alvarez-Vasquez F, Gonzalez-Alco C, Torres NV. Metabolism of citric acid production by Aspergillus niger: Model definition, steady-state analysis and constrained optimisation of citric acid production rate. Biotechnol Bioeng. 2000;70:82-108. [ Links ]
9. Jianlong W. Enhancement of citric acid production by Aspergillus niger using n-dodecane as an oxygen-vector. Process Biochem. 2000;35:1079-1083. [ Links ]
10. Sayer JA, Gadd GM. Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycol Res. 2001;105:1261-1267. [ Links ]
11. Considine PJ, Hackett TJ, Coughlan MP. Solid-state cultivation of Penicillium capsulatum on beet pulp. Biotechnol Lett. 1987;9:131-134. [ Links ]
12. Marier JR, Boulet M. Direct determination of citric acid in milk with improved pyridine-acetic anhydride method. J Dairy Sci. 1958;41:1683-1692. [ Links ]
13. Legiša M, Mattey M. Changes in primary metabolism leading to citric acid overflow in Aspergillus niger. Biotechnol Lett. 2007;29:181-190. [ Links ]
14. Legiša M, Kidrič J. Initiation of citric acid accumulation in the early stages of Aspergillus niger growth. Appl Microbiol Biotechnol. 1989;31:453-457. [ Links ]
15. Legiša M, Cimerman A, Sterle M. Germination of Aspergillus niger in high citric acid yielding medium. FEMS Microbiol Lett. 1981;11:149-152. [ Links ]
16. Legiša M, Mattey M. Glycerol synthesis by Aspergillus niger under citric acid accumulating conditions. Enzyme Microb Technol. 1986;8:607-609. [ Links ]
17. Röhr M, Kubicek CP, Zehentgruber O, Orthofer R. Accumulation and partial re-consumption of polyols during citric acid fermentation by Aspergillus niger. Appl Microbiol Biotechnol. 1987;27:235-239. [ Links ]
18. Röhr M, Kubicek CP. Regulatory aspects of citric acid fermentation by Aspergillus niger. Process Biochem. 1981;16:34-37. [ Links ]
19. Legiša M, Mattey M. Glycerol as an initiator of citric acid accumulation in Aspergillus niger. Enzyme Microb Technol. 1986;8:258-259. [ Links ]
20. Ruijter GJG, Panneman H, Visser J. Overexpression of phosphofructokinase and pyruvate kinase in citric acid-producing Aspergillus niger. Biochem Biophys Acta. 1997;1334:317-326. [ Links ]
21. Papagianni M, Wayman F, Mattey M. Fate and role of ammonium ions during fermentation of citric acid by Aspergillus niger. Appl Environ Microbiol. 2005;71:7178-7186. [ Links ]
22. Kristiansen B, Sinclair CG. Production of citric acid in batch culture. Biotechnol Bioeng. 1978;20:1711-1722. [ Links ]
23. Drever JJ, Stillings LL. The role of organic acids in mineral weathering. Colloids Surf A Physicochem Eng Asp. 1997;120:167-181. [ Links ]
24. Brantley SL. Reaction kinetics of primary rock-forming minerals under ambient conditions. Treat Geochem. 2004;5:73-117. [ Links ]
25. Scott AD, Smith SJ. Susceptibility of interlayer potassium in micas to exchange with sodium. Clays Clay Miner. 1966;14:69-81. [ Links ]
 Correspondence to:
Correspondence to:
Peter Williams
Postal address:
Department of Chemical Engineering, University of Pretoria
Pretoria 0002, South Africa
email:peterjohn.williams@up.ac.za
Received: 07 Sept. 2009
Accepted: 20 Jan. 2010
Published: [To be released]
This article is available at: http://www.sajs.co.za













