Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840Xversão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
https://doi.org/10.17159/0379-4350/2023/v77a16
RESEARCH ARTICLE
Volumetric and viscometric studies of binary mixtures of methanol and some alkyl acetates at varying temperatures
Chukwuebuka M. Ekezie*; Grace A. Cookey; Jane N. Maduelosi
Department of Chemistry, Rivers State University, Port Harcourt, Rivers State, Nigeria
ABSTRACT
The degree of molecular interactions between mixed solvent molecules and thus ideal behavior results from the molecular architecture and chemical nature of component solvents. This understanding is essential in the design and applications of both pure and mixed solvent systems. Density and viscosity measurements of pure and binary mixtures of methanol and n-methyl acetate, n-butyl acetate, and n-pentyl acetate were carried out at different compositions of methanol and temperatures of 25, 30, 35, 40 °C. The experimental data obtained were correlated to the Redlich-Kister equation from where the excess functions; excess molar volumes, excess viscosities, excess Gibbs free energies of activation for viscous flow, fitting coefficients and standard deviations were obtained. The viscosity values were correlated with Hind et al, Kendral and Monroe, Grunberg and Nissan and Frenkel semi empirical models to ascertain the best fit for the systems. The results obtained have been discussed in terms of the structural differences and nature of the interactions between molecules of the mixed solvents.
Keywords: density, excess functions, semi-empirical models, viscosity
INTRODUCTION
Thermodynamic properties of liquid mixtures have attracted considerable interest from scholars due to the vast applications in industries and chemical laboratories.1 The thermodynamics of organic solvents and non-electrolyte liquids is particularly important in the areas of heat transfer, mass transfer as well as chemical separations.2 Experimental determination and study of the physical properties such as density, electric conductivity, and viscosity of substances are very important in the field of chemistry, engineering, petroleum exploration, agriculture, and in the designing of instruments.3,4,5
The study of changes in thermodynamic properties of mixtures and the degree of deviation from ideality gives excellent information on the molecular structure and intermolecular interactions in solvent mixtures.6 Such intermolecular interactions between solvent mixtures are a reflection of the degree of deviation from ideality which gives rise to excess properties. These deviations are associated with synergy between mixture components and are attributed to differences in the chemistry of the individual solvents, experimental conditions, such as temperature differences, and mixing ratios of mixture components.7, 8
Alcohols are polar solvents with high boiling points attributed to self-association arising from hydrogen bonding present in the molecule.9 Polarity and size of molecules are reported to be the predominant factors that determine the magnitude of deviations of mixed solvents from ideal behavior and thus the excess thermodynamic properties of such mixtures10 Specific interactions arise from structural differences and charge transfers.11
Methanol is the simplest known alcohol. In the laboratory, it is majorly used as an analytical solvent for extractions.12 In the industry, methanol is used as basic stock in the production of plastics, enamels13 antifreeze, fuel additives, etc.14,15
Alkyl acetates (esters) are polar aprotic compounds with dipole interactions present as the predominant intermolecular interactions.16 They have medical, biological, biochemical, chemical, and industrial applications.17, 18
Although alcohols and esters have variety of applications in industries, laboratories, pharmaceuticals, and medicine, they may have been studied individually or as mixed solvents with other organic or electrolytic solvents, but to the best of our knowledge, data for the binary mixtures of the chosen solvents in this study appear to be scarce in the literature. Some literature on binary mixtures reviewed in the course of carrying out this research is presented in Table 1.
Thus, the need for an extensive study of the thermodynamic properties as well as theoretical behavior of these binary mixtures. In this research work, we measured the density and viscosity of the pure and binary mixtures of the solvents at vary compositions and temperature. The excess functions were calculated and correlated using the Redlich-Kister equation and semi empirical equations used to analyze the experimental data to theoretical results.
MATERIALS AND METHODS
Methyl acetate (MA), butyl acetate (BA), and methanol (M) with 99% minimum assay were products of Loba chemie PVT Ltd, India. Pentyl acetate (PA), with 95% minimum assay was from Kermel Chemicals, Turkey. Purities of the pure solvents were further assessed by measuring the densities at 30 °C and the values were comparable with those of the literature at the same temperature. Mixtures were prepared by mass in the range of 0 to 1 mole fraction of methanol at interval of 0.1 and store in an air tight glass bottle before use to avoid contamination. All solvents and solvent mixtures were weighed using AE 223 electronic balance with a precision of ±0.1 mg. The uncertainty in mole fraction was approximated to ±0.0001. Densities of pure and binary mixtures were measured using a 10 ml borosilicate glass pycnometer with a uniform capillary stopper. Calibration of the pycnometer was done using distilled water at 25 °C. Temperatures were controlled using electronical thermostatic water bath and monitored with mercury levels in glass thermometer. Viscosities of the pure solvents and binary mixtures were measured using a 20 ml capillary Oswald viscometer with a known viscometer constant (k) of 0.03. The viscometer was calibrated with distilled water at 25 °C to validate the value of k. An electronic stop watch with uncertainty of ±0.01 seconds was used to obtain the flow time. Triplicate measurements of all experiments were done and an average of the readings taken.
RESULTS AND DISCUSSION
Experimental result analysis
The excess molar volumes were calculated from the experimental density values with the equation;
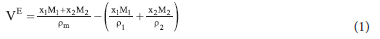
where Ve is the excess molar volume, pm is density of the solvent mixtures, Xj and X2, Mj and M2, Pi and p2 are the mole fractions, molar masses and densities of solvents 1 and 2 respectively.
Excess viscosities of the binary mixtures were calculated using equation 2;

where N1 and N2 are viscosities of the individual solvents and Nm is viscosity of the binary mixtures.
Excess Gibbs free energy of activation for viscous flow of the binary systems was calculated using equation 3;
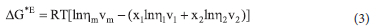
where R is the universal gas constant and T, the absolute temperature.
The values of the excess molar volumes, viscosities and Gibbs free energies of activation for viscous flow were fitted to the Redlich-Kister polynomial type equation by least-squares fitting (Eq. 4).

n is the number of coefficients and Ai, the optimum number of coefficient determined from the examination of the variation of standard deviation as calculated using equation 5
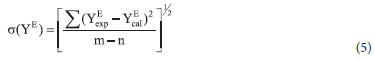
where m is the number of experimental data points and n is the number of coefficients. The subscripts exp. and cal. represent the experimental and calculated values of any of the excess parameters.
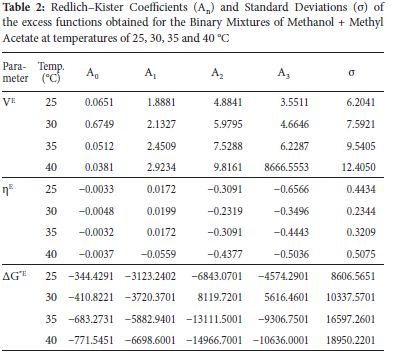
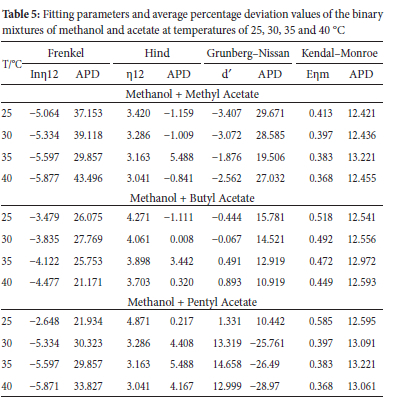
Values of Redlich-Kister coefficients (An) and standard deviation (a) for all binary systems studied are presented in Tables 2, 3 and 4. Methanol + methyl acetate system gave lower standard deviation (a) than methanol + butyl acetate and methanol + pentyl acetate systems.
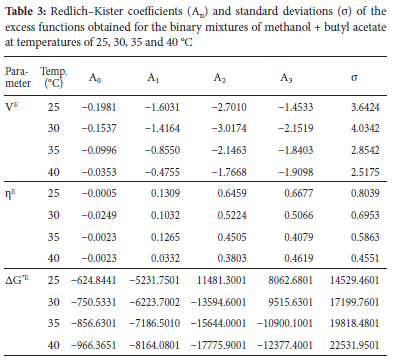
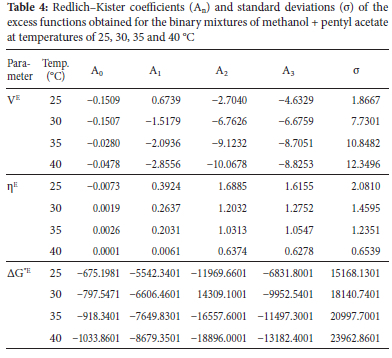
Plots of excess molar volumes (Ve) against mole fractions of methanol (X1) for methanol and methyl acetate (M+MA), butyl acetate (M+BA) and pentyl acetate (M+PA) systems at varying temperatures (25, 30, 35 and 40 °C) are presented in Figure 1.
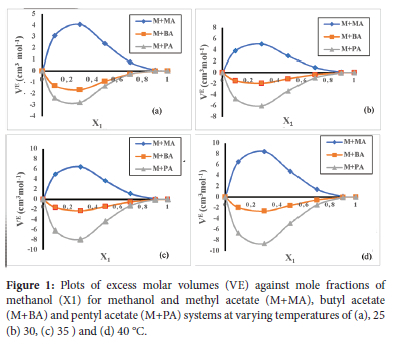
From the results obtained, Ve were found to be positive in methanol + methyl acetate mixtures but negative in methanol + butyl acetate and methanol + pentyl acetate mixtures at the different mole fractions of methanol and temperatures studied. Positive deviations observed in the methanol + methyl acetate mixtures suggest volume expansion in the binary system. Positive VE values are usually attributed to weak intermolecular interaction or repulsive forces between unlike molecules. On mixing, methyl acetate appears to disrupt the self-association of methanol hydrogen bonds leading to rupturing of the bonds and hence, the observed positive deviation. Positive deviation may also be attributed to weak dipole-dipole interaction dominant in the mixed system. The negative Ve values in methanol + butyl acetate and pentyl acetate suggest volume contraction in the binary systems. It is possible that on mixing, methanol formed new hydrogen bonds with the dipolar oxygen of the esters. This behavior may be attributed to inductive effect which increased the electron density and thus, the proton accepting ability of the alkyl acetate. Increase in alkyl chain length of acetates increase the inductive effect. Hence, the observed trend in -Ve values methanol + pentyl acetate > methanol + butyl acetate > methanol + methyl acetate. Raju et al., reported similar results.19 The degree of deviation increased with increase in temperature as seen in the plots. This observation is due to the increase in entropy and dominance of packing effect. This is in line with Rajalakshmi et al. observation.20
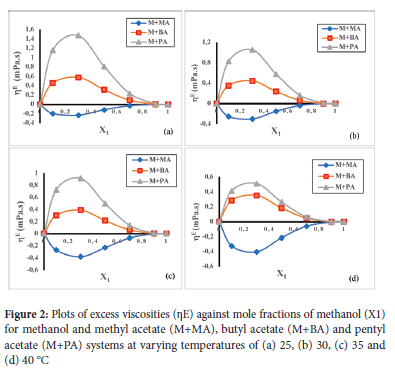
Plots of deviation in excess viscosity (nE) against mole fraction at 25, 30, 35 and 40 °C for methanol + methyl acetate, methanol + butyl acetate and methanol + pentyl acetate are presented in Figure 2 (a-d). nE values were found to be negative for methanol + methyl acetate and positive for methanol + butyl acetate and methanol + pentyl acetate systems. Negative nE suggest that the binary system is less viscous than the pure solvents. Weak intermolecular interactions have been reported by Shaik et al. to be responsible for negative excess molar volume.21 Negative nE may be attributed to dissociation of dipolar acetates and rupturing of associated hydrogen bonds of the methanol. Negative nE may also be due to the presence of weak dipole-dipole interaction contributed by methyl acetate. The positive deviations observed for methanol + butyl acetate and pentyl acetate suggest that the binary system were more viscous than the pure solvents. Positive deviations also suggest that there was strong intermolecular interaction arising from hydrogen bonding between unlike molecules of methanol and the esters. Similar reports were presented by Pikkaraine,22 Furthermore, the deviation in viscosity may also be accounted for in terms of difference in molar volume of the individual solvents which favoured the accommodation of smaller molecules by the larger ones. At 25 °C, pentyl acetate and butyl acetate with molar volumes of 150.64 mol dm-3 and 133.49 mol dm-3 respectively tend to accommodate methanol (with molar volume = 40.71 mol dm-3) better than methyl acetate whose molar volume is 80 mol dm-3. The magnitude of deviation in viscosities decreased as temperature increased. The observed decrease in nE may be attributed to increase in thermal energy which may oppose attractive force binding the molecules together. Seeton, made similar observation.23
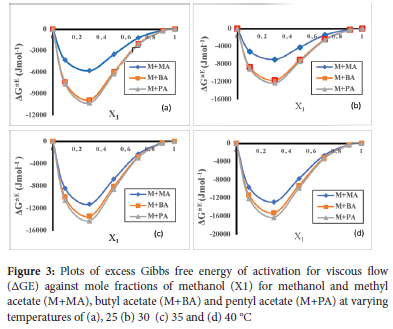
Plots of deviation in excess Gibbs Free Energy of activation for viscous flow (ΔG*E) against mole fraction at 25, 30, 35 and 40 °C for methanol + methyl acetate, methanol + butyl acetate and methanol + pentyl acetate are presented in Figure 3 (a-d). ΔG*E were found to be negative in all studied binary systems. Negative ΔG*E values suggest spontaneous mixing and presence of weak intermolecular interaction between unlike molecules. As temperature increased, the magnitude of negative ΔG*E increased and this could be attributed to increase in entropy that accompanies increase in temperature and thus increased spontaneity. This is in line with results published by Tuck.24
Theoretical analysis
Experimental viscosity data were further correlated with semi-empirical models (Frenkel, Hind, Grunberg-Nissan and Kendral-Monroe; equations 6, 8, 9 and 11, respectively).
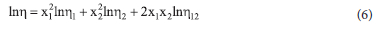
where n is expressed as follows:

where lnn12 is the Frenkel correlation parameter and n12 is the Hind correlation parameter

The dynamic viscosities were also tested by the Grunberg-Nissan equation;
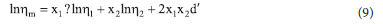
where d' is an interaction parameter proportional to energy interchange which is also a function of temperature of the binary mixtures.

Equation 10 is referred to as the Kendall-Monroe cube-root equation. The right-hand side of equation 10 has been multiplied by the product of the mole fractions to obtain equation 11.

where Enm is the Kendral-Monroe correlation parameter
The correlating ability of equation 6, 8, 9 and 11 were tested by calculating the Average Percentage Deviations (APD) between the experimental and the calculated viscosities using equation.

where nexptal and ncalc represent the experimental and calculated viscosities respectively, N is the number of experimental data points.
The results of viscosity semi empirical calculations and average percentage deviations are presented in Table 5. The use of semi empirical models in multicomponent mixtures has been reported Dikio,25 to provide the following information:
1. They identify some models as convenient reference for the interpretation of observed deviations.
2. They help in suggesting models that best describe the characteristics of the systems.
3. They also predict the parameter that should be improved when the models have multiple contributors.
The Grunberg-Nissan interactive parameter (d') has been reported by Shaik et al. to be a good predictive yardstick for the type of interaction in mixed systems.21 Positive d' values are ascribed to specific interaction and negative d' values are described in terms of weak intermolecular interactions present in the system. The value of d' for methanol + methyl acetate was negative at all temperatures and solvent compositions, indicating the presence of weak interaction. As the alkyl chain of the acetate group increased the value of d' appreciated to positive values. The d' parameter for methanol + butyl acetate and methanol was positive at all temperatures and solvent composition, indicating formation of new hydrogen bonds between unlike moles and interstitial fitting of molecules as the predominant factors in the systems. The APD values for Kendrall-Monroe model have a minimal uniform positive variation at all temperatures and this suggest that the model describes the system better than other models as suggested by Kemeakagha.26
CONCLUSIONS
The excess properties of binary mixtures of methanol + methyl acetate, methanol + butyl acetate and methanol + pentyl acetate have been determined at 25, 30, 35 and 40 °C over the entire range of methanol compositions. Excess molar volumes were found to be positive for methanol + methyl acetate mixtures and negative for methanol + butyl acetate and methanol + pentyl acetate mixtures over the entire compositions and temperatures. Excess viscosities were negative for methanol + methyl acetate mixture but positive for methanol + butyl acetate and methanol + pentyl acetate binary mixtures. Excess molar volumes and excess viscosities were attributed to weak dipole-dipole interaction, rupturing of hydrogen bond associates, formation of hydrogen bonds between unlike molecules and geometrical fitting of molecules. Gibbs Free Energies of activation for viscous flow were found to be negative and deviations were discussed in terms of spontaneity of binary systems. It was observed that the magnitude of interaction increased with increase in carbon chain length of the acetates. Grunberg-Nissan and Kendrall-Monroe models were also identified as best semi empirical models for the systems. The properties of the mixed solvent systems studied exhibited better interactive properties than those of the pure solvents for methanol + butyl acetate and pentyl acetate binary mixtures and will give better industrial applications while the methanol + methyl acetate mixture had a lesser interactive property as compared with the molecules of pure solvents.
ACKNOWLEDGEMENTS
Authors are grateful to the HOD of chemistry, Rivers State University for granting us access to all facilities and instruments used in this research. We also wish to appreciate Dr JA Kemeakegha for his assistance in explaining the Redlich-Kister equation.
ORCID IDS
CM Ekezie: https://orcid.org/0009-0003-6897-0871
GA Cookey: https://orcid.org/0000-0003-0199-9613
NJ Maduelosi: https://orcid.org/0000-0003-3473-3678
REFERENCES
1. Elliot RJ, Lira CT. Introductory chemical engineering thermodynamics. 2nd ed. New Jersey, America, Pearson Education Incorporated, Prentice Hall; 2012. [ Links ]
2. Deshwal BR, Sharma A, Singh KC. Speed of sound and excess isentropic compressibilities of butyl acetate + aromatic hydrocarbons. Chin J Chem Eng. 2008;16(4):599-604. https://doi.org/10.1016/S1004-9541(08)60127-5 [ Links ]
3. Ahmad KS, Ghotbi C, Taghikhani V, Jalili A. Correlation of viscosity of aqueous solutions of alkanolamine mixtures based on the eyring's theory and Wong-Sandler mixing rule. Iran J Chem Chem Eng. 2013;32(2):9-17. https://doi.org/10.30492/ijcce.2013.5863 [ Links ]
4. Maduelosi NJ, Bagshaw AP, Ekezie CM. Molar conductivities, salinity and total dissolved solids of KBr and KCl in aqueous solution. Acad J Chem. 2017;2(7):61-64. [ Links ]
5. Boisa N, Odagwe BU. Indoor dust-based pollution status and risk assessment for a rural town, Ebedei in Nigeria hosting gas flare facility. J Environ Prot (Irvine Calif). 2019;23(2):208-220. https://doi.org/10.4236/jep.2019.102012 [ Links ]
6. Lakshmi BJ, Gowrisankar M, Rambabu C, Ramachandran D. Volumetric ultrasonic and viscometric studies of binary liquid mixtures of n-ethyl aniline + chlrobenzene, + bromobenzene, + 1, 2-dichchlorobenzene + 1, 3-dichlorobenze + 1, 2, 4-trichlorobenze at 303.15 and 308.15 K. Korean J Chem Eng. 2014; 31(5): 881-895. https://doi.org/10.1007/s11814-013-0235-0 [ Links ]
7. Altuwaim MS, Alkhaldi KHAE, Al-Jimaz AS, Mohammad AA. Physico-chemical properties of binary mixtures of N. N-dimethlformamide with 1-octanol, 1-nonanol and 1-decanol at different temperatures. J Chem Thermodyn. 2013;58:367-376. https://doi.org/10.1016/JJCT.2011.12.002 [ Links ]
8. Venkatramana L, Srevivasulu, K, SivaKumar, K, Reddy D. Thermodynamic properties of binary mixture containing 1-alkanols. J Therm Anal Calorim. 2014;115(2):1829-1834. https://doi.org/10.1007/s10973-013-3473-9 [ Links ]
9. Finar IL. Organic Chemistry Vol 1, 6th ed. Pearson, India (2012). [ Links ]
10. Shimomura T, Fujii K, Takamuku T. Effects of the alkyl-chain length on the mixing state of imidazolium-based ionic liquid-methanol solutions. Phys Chem Chem Phys. 2010;12(38):12316-12324. https://doi.org/10.1039/c0cp00614a [ Links ]
11. Gowrisankar M, Sivarambabu S, Venkateswarlu P, Kumar KS. Excess volumes, speed of sounds, isentropic compressibilities and viscosities of binary mixtures of N-ethylanile with some aromatic ketones at 303.15 K Bull Korean Chem Soc. 2012;33(5):1686-1692. https://doi.org/10.5012/BKCS.2012.33.5.1686 [ Links ]
12. Mahdi-Pour B, Jothy SL, Latha LY, Cheng Y, Sasidharan S. Antioxidant activity of methanol extract of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012;2(12):960-965. https://doi.org/10.1016/S2221-1691(13)60007-6 [ Links ]
13. Mokhatab S, Poe WA, Mak JY. Handbook of natural gas transmission and processing: principles and practices. Gulf Professional Publishing; 2018. [ Links ]
14. Ali A, Nain AK, Sharma VK, Ahmad S. Molecular interaction in binary mixtures of tetrahydrofuran with alkanols (C6, C8, C10): an ultrasonic and volumetric study. Indian J Pure Appl Phy. 2004; (42): 666-673. [ Links ]
15. Khadzhiev SN, Kolesnichenko N, Ezhova NN. Slurry technology in methanol synthesis. Petrol Chem. 2016; 56(2):77-95. https://doi.org/10.1134/S0965544116020079 [ Links ]
16. Bettelheim FA, Brown WH, Campbell MK, Farrell SO, Torres O. Introduction to general, organic and biochemistry. 10th ed. America, Cengage Learning. 550 (2012). [ Links ]
17. O'Neil MJ. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. 15th ed. Cambridge, UK, Royal society of Chemistry; 2013. [ Links ]
18. Bahadur I, Deenadayalu N, Ramjugernath D. Effects of temperature and concentration on interactions in methanol + ethyl acetate and ethanol + methyl acetate or ethyl acetate systems: insights from apparent molar volume and apparent molar isentropic compressibility study. Thermochim Acta 2014;10(577):87-94. https://doi.org/10.1016/j.tca.2013.12.016. [ Links ]
19. Raju R, Ravikumar S, Arokiaraj RG, Karlapudi S, Sivakumar K, Pandiyan V. Excess thermodynamic properties and ftir studies of binary of 1, 3-dichlorobenzene with alkyl acetates (C1-C5) at different temperatures. Chem Data Collect. 2020;9:100504. https://doi.org/10.1016/j.cdc.2020.100504 [ Links ]
20. Rajalakshmi R, Ravikumar S, Gaba R, Pandiyan V. Thermodynamic properties and IR studies of binary mixtures of benzyl amine with alkyl esters at different temperatures. Chem Data Collect. 2019;24:100278. https://doi.org/10.1016/j.cdc.2019.100278 [ Links ]
21. Shaik J, Sankar MG, Ramachandran D, Rambabu C. Orientation effect on sign and magnitude of excess thermodynamic functions of non electrolyte solutions at different temperatures (303.15 K, 308.15 K, and 313.15 K). Korean J Chem Eng. 2014;31(8):1460-1469. https://doi.org/10.1007/s11814-014-0088-1 [ Links ]
22. Pikkarainen L. Densities and viscosities of binary mixtures of N, N-dimethylacetamide with aliphatic alcohols. J Chem Eng Data. 1983;28(3):344-347. https://doi.org/10.1021/JE00034A009 [ Links ]
23. Seeton CJ. Viscosity-temperature correlation for liquids. International Joint Tribology Conference. 2013;42592:131-142. https://doi.org/10.1007/s11249-006-9071-2 [ Links ]
24. Tuck AF. Gibbs free energy and reaction rate acceleration in and on microdroplets. Entropy (Basel). 2019;21(11):1044. https://doi.org/10.3390/e21111044 [ Links ]
25. Dikio ED. Derived thermodynamic properties of binary mixtures of M-xylene, O-xylene, and P-xylene, with N, N-dimethylformamide at T=(293.15, 303.15, 313.15 and 323.15)K. Orient J Chem. 2014;30:953-967. https://doi.org/10.13005/ojc/300306 [ Links ]
26. Kemeakegha AJ, Jumbo BA. Thermodynamic study on density and viscosity of binary mixtures of ethyl acetoacetate with (C4-C9) aliphatic ketones at (303.15 and 308.15.)K. Int J Innov Sci Res Technol. 2020;5(6):1598-1614. https://doi.org/10.38124/IJISRT20JUN1107 [ Links ]
Received 27 March 2023
Revised 29 June 2023
Accepted 18 July 2023
* To whom correspondence should be addressed: Email: chukwuebuka.ekezie1@ust.edu.ng













