Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
African Journal of Laboratory Medicine
versión On-line ISSN 2225-2010
versión impresa ISSN 2225-2002
Afr. J. Lab. Med. vol.13 no.1 Addis Ababa 2024
http://dx.doi.org/10.4102/ajlm.v13i1.2373
BRIEF REPORT
Diagnostic cut-off value of haemoglobin A1c for diabetes mellitus in Harare, Zimbabwe
Chido W. BvumbiI; Vinie KouamouII, III; Ngalulawa KoneIV, V; Trust ZaranyikaII; Lloyd BoworaI; Hilda T. MatariraI; Raylton P. ChikwatiIV, VI
IDepartment of Laboratory Diagnostic and Investigative Sciences, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
IIDepartment of Primary Care and Health Sciences, Internal Medicine Unit, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
IIIBiomedical Research and Training Institute, Harare, Zimbabwe
IVDepartment of Chemical Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VDepartment of Chemical Pathology, National Health Laboratory Service, Johannesburg, South Africa
VISydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Very little is known about the diagnostic performance of the American Diabetes Association glycated haemoglobin (HbA1c) cut-off of 6.5% in resource-limited settings. This study, conducted between February 2023 and May 2023, aimed to determine the optimal HbA1c cut-off for the diagnosis of diabetes mellitus by measuring HbA1c and fasting plasma glucose levels in 120 adults attending care at a tertiary hospital in Harare, Zimbabwe. The optimal HbA1c cut-off was 6.1% and glucose levels were strongly correlated with HbA1c values. The prevalence of diabetes mellitus was higher (28.3%) at our derived HbA1c cut-off than with the American Diabetes Association criterion (21.6%).
WHAT THIS STUDY ADDS: This study highlights the need for population-specific cut-off HbA1c values in the diagnosis of diabetes mellitus.
Keywords: glycated haemoglobin; diabetes mellitus; fasting plasma glucose; receiver operating characteristics curves; Zimbabwe.
Introduction
The prevalence of diabetes mellitus (DM) in Zimbabwe has increased from 0.4% (95% confidence interval [CI]: 0.0-1.9) before 1980, to 5.7% (95% CI: 3.3-8.6) after 1980.1 While the reasons behind these changes are multifactoral, a key factor could be the transition in the diagnostic criteria of DM from urine to blood testing. From the eight studies evaluated, three used urine for glucose testing and were all conducted before 1980.1 When comparing the prevalence between these studies, it is apparent that the transition from urine to blood testing had an impact on the observed increase. It is therefore important to evaluate the diagnostic criteria in this population to improve diabetic care.
Currently, a glycated haemoglobin (HbA1c) of 6.5% is used for diagnosing DM, as guided by the American Diabetes Association (ADA).2 However, the diagnostic cut-off value of HbA1c has been shown to vary across different races and ethnicities.3 Studies have shown that HbA1c levels are higher in African Americans, African Brazilians, British African Caribbeans, Asians and Latinos than in populations of European ancestry.4,5 Given this evidence, a universal HbA1c cut-off may underdiagnose DM in some populations. We therefore aimed to determine an optimal HbA1c cut-off value for DM in a black Zimbabwean population of African ancestry.
Methods
Ethical considerations
This study was approved by the Joint Research Ethics Committee of the University of Zimbabwe (JREC 48/2023) and the Parirenyatwa Group of Hospital's Clinical Director. Informed written consent was obtained from all participants. To protect privacy and confidentiality, all collected blood specimens were de-identified to ensure that no individual could be linked to the study information. All data were captured on a password-protected Excel spreadsheet (Microsoft Corp., Redmond, Washington, United States) and printed results were stored in a cabinet only accessible to the study investigators.
Study design
In this cross-sectional study, we recruited adults (≥ 18 years) from Harare, Zimbabwe, with a body mass index ≥ 25 kg/m2. Recruitment of study participants was done from 15 February 2023 to 14 May 2023 at the Diabetic Clinic, Parirenyatwa Group of Hospitals, a tertiary hospital. A minimum sample size of 119 participants was calculated using the Dobson formula. Participants who self-reported pregnancy or had anaemia, renal disorders or haemoglobinopathies based on a previous diagnosis by a healthcare practitioner were excluded.
Specimen processing
Blood specimens were collected by trained nurses and only used for this study. From each participant, approximately 8 mL of blood were collected after an 8 h overnight fast. Participants were advised to have their dinner before midnight and not ingest any food or drink, except for water, until the next day when blood was drawn. Blood specimens were collected in sodium fluoride- and ethylenediamine tetraacetic acid-containing vacutainer tubes (Becton, Dickinson and Company, Plymouth, United Kingdom). All blood specimens were transported from the clinic to the Interpath Medical Laboratories, where they were processed, analysed and stored.
At the laboratory, blood specimens in sodium fluoride tubes were immediately centrifuged at 3500 revolutions per minute for 5 min (Yingtai Instrument, Changsha City, China). Testing of fasting plasma glucose (FPG) was done within 2 h from blood collection. Ethylenediamine tetraacetic acid blood specimens were stored at 2 °C - 8 °C before transportation on dry ice to the National Health Laboratory Service in Johannesburg, South Africa, for HbA1c measurement.
Anthropometric variables
Body weight was measured using a 120 kg scale (MCP Camry, Medicare Products Inc., New Delhi, India). Participants were asked to wear light clothing before standing on the scale. Standing height was measured using a wooden board which had a sliding headpiece (United Nations Children's Fund, New York, New York, United States). Participants were asked to maintain a straight posture against the wall before a reading was taken. Body mass index was calculated as a ratio of the measured weight in kg and height in m2.
Fasting plasma glucose
Fasting plasma glucose levels were measured using the hexokinase methodology on a Mindray analyser (Mindray BS200, Shenzhen, China). Mindray reagents, calibrators, and quality control materials were used. Diabetes mellitus was defined as a FPG ≥ 7.0 mmol/L.2
Glycated haemoglobin
HbA1c levels were measured in ethylenediamine tetraacetic acid whole blood using a Bio-Rad High-Performance Liquid Chromatography analyser, reagents, calibrators, and quality control materials (Bio-Rad VARIANT II, Montreal, Quebec, Canada).
Statistical analyses
All statistical analyses were performed using Stata 16.1 (StataCorp LP, College Station, Texas, United States). Continuous variables were expressed as mean ± standard deviation if data followed a Gaussian distribution, or median and interquartile range (IQR) if data were skewed. Continuous variables were compared using the Student's t-test and Mann-Whitney U test, as appropriate. Pearson correlation was used to assess the relationship between FPG and HbA1c levels. Statistical significance was set at p < 0.05. Receiver operator characteristic curves were plotted to identify the diagnostic cut-off for HbA1c using the highest Youden's J index.6 Sensitivity and specificity were calculated for both the derived and ADA HbA1c cut-off values.
Results
Characteristics of study population
We recruited a total of 120 participants, comprising 62 women and 58 men, from a pool of 138 eligible participants. Non-participation was primarily due to the inability to commute to the study clinic and failure to provide a fasting blood specimen. Their mean (± standard deviation) age was 43.2 (±14.0) years. Median (IQR) body mass index was 29.0 (26.5-31.3) kg/m2. Body mass index was higher in women than men. No other differences were observed (Table 1).
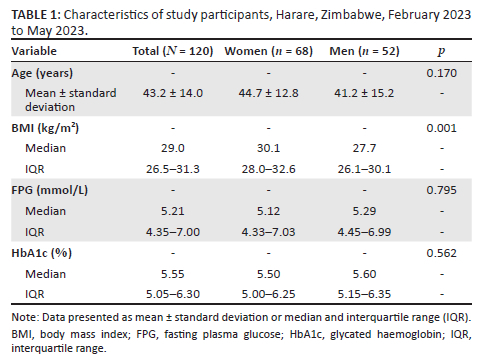
Fasting plasma glucose and HbA1c
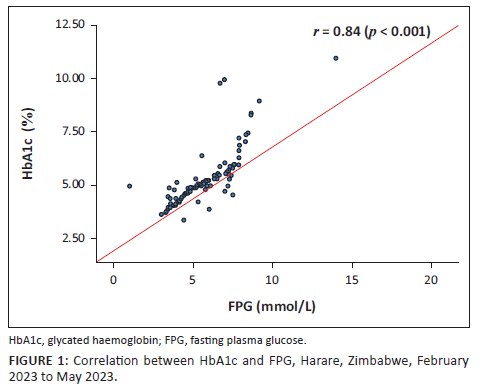
The median (IQR) FPG levels were 5.21 (4.35 - 7.00) mmol/L and HbA1c, 5.55% (5.05% - 6.30%) (Table 1). When the ADA criterion (FPG ≥ 7.0 mmol/L) was applied, 31 (26.0%) participants had DM. Their median (IQR) FPG levels were 7.50 (7.23 - 8.12) mmol/L and HbA1c, 6.80% (6.35% - 8.40%) (data not shown). Furthermore, there was a linear relationship between FPG and HbA1c levels (r = 0.84, p < 0.001) (Figure 1).
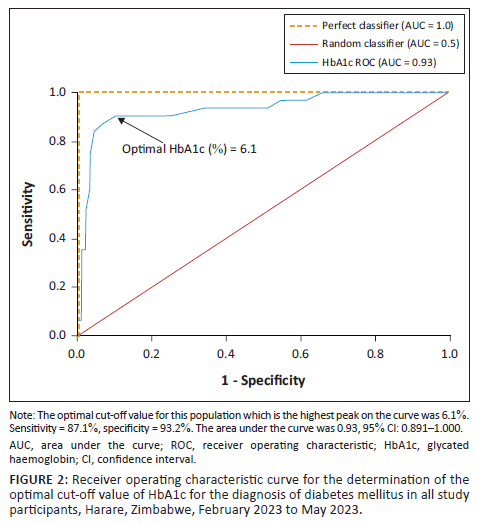
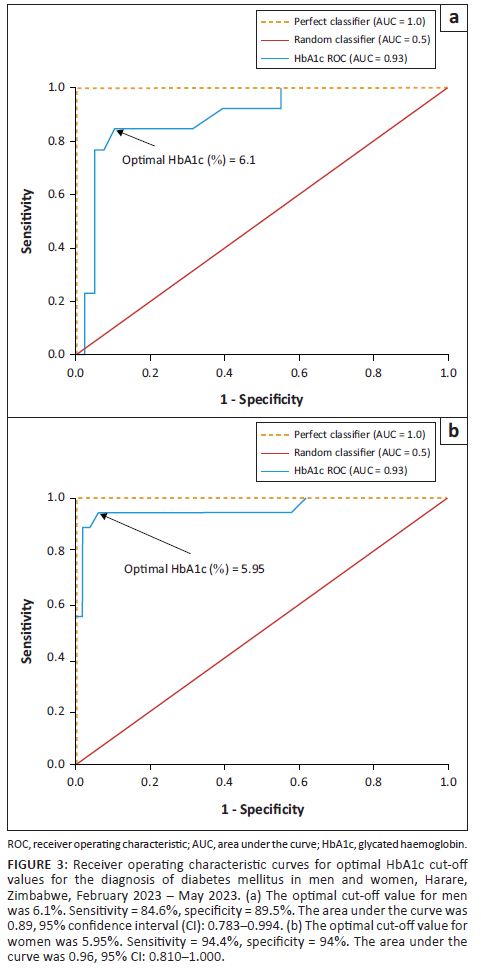
For all participants, the optimal HbA1c cut-off for DM was 6.10% (Figure 2). When stratified according to sex, the optimal HbA1c cut-off was unchanged in men, at 6.10%, but lower in women, at 5.95% (Figure 3).
Diagnostic performance
The derived HbA1c cut-off of 6.1% had a higher sensitivity (87.1%) than the ADA criterion (71.0%) (Online Supplementary Table 1). However, specificity was slightly higher in the ADA criterion (95.5% vs 93.2%). The prevalence of DM was higher (28.3%) when the 6.1% cut-off was applied, but lower with the ADA criterion (21.6%) (Online Supplementary Table 1).
Discussion
Our results showed that a 6.1% HbA1c cut-off had high sensitivity and specificity in black Zimbabwean participants with body mass index ≥ 25 kg/m2. When we applied the ADA criterion, the sensitivity decreased, and we identified 6.7% fewer diabetics.
In support of our findings, a meta-analysis from 2022 including seven African studies showed that the sensitivity for HbA1c in the diagnosis of DM was highest (74.9%) at 6.0%.7 Two global meta-analyses showed that the 6.5% cut-off was associated with lower sensitivity (59.4% and 52.7%).8,9 Other studies from South Africa (6.1%),10 India (6.1%),11 and Haiti (6.3%)12 have also shown lower HbA1c cut-offs than the ADA criterion.
Our findings and others' indicate that the optimal Hb1Ac cut-off differs from the ADA criterion. Some factors, such as Hb1Ac analytical methods and haemoglobin structure, may contribute to these differences.13 Furthermore, different inclusion criteria may contribute to heterogeneity between studies.14,15
We also observed a positive linear relationship between FPG and HbA1c levels. These findings are consistent with a meta-analysis conducted in 2015 which reported a similar trend (r = 0.74, p < 0.001).16
The slightly lower HbA1c cut-off found in our study, of 5.95% in women compared to 6.10% in men, has not been reported previously. However, studies have shown that black women of African ancestry have lower hepatic clearance of insulin, differential ß-cell responsiveness and therefore higher mean blood glucose levels than white women of European ancestry.17,18 However, there is scarcity of data comparing black women and black men.
Strengths of our study include using the high-performance liquid chromatography method and recruiting from one urban population, where lifestyle and environmental factors were likely to be homogenous. In addition, all specimens were collected within the same weather season (autumn), thus the seasonal influence on HbA1c was minimal.
Limitations
Limitations of our study include its small sample size, and sampling from a tertiary clinic; therefore, findings may not be generalisable. Additionally, due to limited resources, we did not have specific symptomology, such as diabetic retinopathy, to confirm DM. We only collected data on body mass index, but not other risk factors that include family history, dietary intake, alcohol consumption, and hypertension. These could have been valuable in assessing the impact of the diagnostic performance of HbA1c against FPG. Another limitation was that we relied on ordered fasting which could not be standardised since participants would present at the study clinic after an overnight fast. It is possible that not all participants were compliant to the instructions given, as some could have misinterpreted that fasting only applies to meals, not beverages and sweets before the test. Furthermore, we did not perform the oral glucose tolerance test, which is known for its higher sensitivity and specificity in the diagnosis of DM.
Conclusion
This study demonstrates a higher diagnostic performance of an HbA1c cut-off value of 6.1% in a black population from Harare, Zimbabwe, and that the ADA cut-off was not optimal for this population. More studies are warranted to confirm these findings in a larger population with better representation of geographical location and more standardised methods of collecting fasting specimens.
Acknowledgements
We would like to acknowledge the study participants and nurses at Parirenyatwa Group of Hospitals. We also thank the staff and management at the Interpath Medical Laboratories and National Health Laboratory Service where the biochemical testing was performed.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
C.W.B., T.Z., L.B. and H.T.M. conceptualised the study. C.W.B. and R.P.C. performed the experimental procedures. N.K. sourced HbA1c reagents. C.W.B., L.B. and R.P.C. performed the data analysis. C.W.B., R.P.C. and V.K. drafted the first version of the manuscript. R.P.C., V.K., L.B., H.T.M., N.K. and T.Z. reviewed intellectual content. All authors approved the final version of the manuscript for submission.
Sources of support
This study did not receive a grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The authors confirm that de-identified raw data supporting the findings of this study are available from the corresponding author, R.P.C., on request.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Mutowo M, Gowda U, Chamunorwa J, Lorgelly P, Owen A, Renzaho A. Prevalence of diabetes in Zimbabwe: A systematic review with meta-analysis. Int J Public Health. 2015;60(1):1-11. https://doi.org/10.1007/s00038-014-0626-y [ Links ]
2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabet Care. 2014;37(SUPPL.1):81-90. https://doi.org/10.2337/dc14-S081 [ Links ]
3.Ford CN, Leet RW, Kipling LM, et al. Racial differences in performance of HbA1c for the classification of diabetes and prediabetes among US adults of non-Hispanic black and white race. Diabet Med. 2020;36(10):1234-1242. https://doi.org/10.1111/dme.13979 [ Links ]
4.Khosla L, Bhat S, Fullington LA HRM. HbA1c performance in African descent populations in the United States with normal glucose tolerance, prediabetes, or diabetes: A scoping review. Prev Chronic Dis. 2021;18(200365):1-16. https://doi.org/10.5888/pcd18.200365 [ Links ]
5.Cavagnolli G, Pimentel AL, Aparecida P, Freitas C, Camargo L. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS One. 2017;12(2):e0171315. https://doi.org/10.1371/journal.pone.0171315 [ Links ]
6.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458-472. https://doi.org/10.1002/bimj.200410135 [ Links ]
7.Chivese T, Hirst J, Matizanadzo JT, et al. The diagnostic accuracy of HbA(1c), compared to the oral glucose tolerance test, for screening for type 2 diabetes mellitus in Africa-A systematic review and meta-analysis. Diabet Med. 2022;39(4):e14754. https://doi.org/10.1111/dme.14754 [ Links ]
8.NCD Risk Factor Collaboration. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: A pooled analysis of 96 population-based studies with 331,288 participants. Lancet Diabet Endocrinol. 2015;3(8):624-637. https://doi.org/10.1016/S2213-8587(15)00129-1 [ Links ]
9.Kaur G, Lakshmi PV, Rastogi A, et al. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: A systematic review and meta-analysis. PLoS One. 2020;15(11):e0242415. https://doi.org/10.1371/journal.pone.0242415 [ Links ]
10.Zemlin AE, Matsha TE, Hassan MS, Erasmus RT. HbA1c of 6.5% to diagnose diabetes mellitus - Does it work for us? - The Bellville South Africa study. PLoS One. 2011;6(8):e22558. https://doi.org/10.1371/journal.pone.0022558 [ Links ]
11.Kumar PR, Bhansali A, Ravikiran M, et al. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: A community-based study. J Clin Endocrinol Metab. 2010;95(6):2832-2835. https://doi.org/10.1210/jc.2009-2433 [ Links ]
12.Exebio JC, Zarini GG, Vaccaro J, Exebio C. Use of hemoglobin A1C to detect Haitian- Americans with undiagnosed type 2 diabetes. Arq Bras Endocrinol Metabol. 2012;56(7):449-455. https://doi.org/10.1590/S0004-27302012000700007 [ Links ]
13.Chen Z, Shao L, Jiang M, Ba X, Ma B, Zhou T. Interpretation of HbA1c lies at the intersection of analytical methodology, clinical biochemistry and hematology (Review). Exp Ther Med. 2022;24(6):707. https://doi.org/10.3892/etm.2022.11643 [ Links ]
14.Holman R. A brief history of the UK Prospective Diabetes Study: Rury Holman, for the UKPDS Group. BJD. 2022;22(Suppl 1):S32-35. https://doi.org/10.15277/bjd.2022.359 [ Links ]
15.The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabet Care. 1987;10(1):1-19. https://doi.org/10.2337/diacare.10.1.1 [ Links ]
16.Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Archiv Public Health. 2015;73:43. https://doi.org/10.1186/s13690-015-0088-6 [ Links ]
17.Chung ST, De La Cruz MG, Aldana PC, et al. Postprandial insulin response and clearance among black and white women: The Federal women's study. J Clin Endocrinol Metab. 2019;104(1):181-192. https://doi.org/10.1210/jc.2018-01032 [ Links ]
18.Cree-Green M. Postglucose hyperinsulinemia in black women is not what we thought. J Clin Endocrinol Metab. 2019;104(2):266-268. https://doi.org/10.1210/jc.2018-02213 [ Links ]
 Correspondence:
Correspondence:
Raylton Chikwati
rayltonc@gmail.com
Received: 15 Nov. 2023
Accepted: 20 Feb. 2024
Published: 23 Apr. 2024
Note: Additional supporting information may be found in the online version of this article as Online Supplementary Document 1.














