Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
African Entomology
versión On-line ISSN 2224-8854versión impresa ISSN 1021-3589
AE vol.32 Pretoria 2024
https://doi.org/10.17159/2254-8854/2024/a16992
RESEARCH ARTICLE
Lethal and sublethal effects of insecticides on Bathycoelia distincta (Heteroptera: Pentatomidae)
Elisa PalI, II; Jeremy D. AllisonI, II, III; Brett P. HurleyI, II; Bernard SlippersII, IV; Gerda FourieII, IV
IDepartment of Zoology and Entomology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa
IIAfrican Centre of Chemical Ecology, Innovation Africa Campus, University of Pretoria, Pretoria, South Africa
IIINatural Resources Canada-Canadian Forest Service, Great Lakes Forestry Centre, Sault Ste Marie, Canada
IVDepartment of Biochemistry, Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa
ABSTRACT
Bathycoelia distincta is one of the most dominant stink bug pests associated with macadamia orchards in South Africa. Understanding the toxicity and sublethal effects of insecticides on this pest is essential for its effective management. This study tested four commercial insecticide formulations, consisting of one organophosphate (acephate) and three pyrethroids (lambda-cyhalothrin, beta-cyfluthrin and tau-fluvalinate). The toxicity of these insecticides and their behavioural effects on mobility were assessed. The sublethal effects of lambda-cyhalothrin on the biological parameters of parent B. distincta (F0) and offspring generations (F1) were also determined by treating B. distincta adults with sublethal concentrations (LC10 and LC30). In toxicity bioassays, acephate was more toxic to B. distincta than lambda-cyhalothrin, beta-cyfluthrin and tau-fluvalinate. Behavioural changes were only observed in bugs exposed to pyrethroids, resulting in an increase in the distance walked and decrease of angular velocity. In the F0 generation, LC30 reduced the fecundity whereas the LC10 and LC30 accelerated development of the F1 generation. These results suggest that pyrethroids may enhance the dispersal of this pest and stimulate the growth of offspring populations. Further experiments should be conducted to confirm these results and understand the mechanism of action of pyrethroids on B. distincta.
Keywords: stink bug, toxicity, pyrethroids, macadamia
INTRODUCTION
Stink bugs (Hemiptera: Pentatomidae) are important pests of various fruit and vegetable crops (McPherson and McPherson 2000; McPherson 2018). Nymphs and adults primarily feed on the reproductive parts of crops including the flowers, seeds, and fruit, although other tissues (e.g., roots, stems, leaves) are also fed on. This feeding results in the stunting of plants and dropping of bolls and fruit and in some cases results in yield losses (McPherson and McPherson 2000; McPherson 2018).
Broad-spectrum insecticides such as organophosphates, pyrethroids and neonicotinoids remain the main strategy for controlling populations of stink bugs (Greene et al. 2018; Sosa-Gómez et al. 2020). Repeated and long-term use of insecticides against stink bugs can result in the development of resistance (Tuelher et al. 2018; Sosa-Gómez et al. 2020). For example, intensive use of pyrethroids and organophosphates on soybean in Brazil reduced the susceptibility of Euschistus heros (Fabricius) considerably, which led to increased concentration of active ingredients in the formulation of some acephate products (Tuelher et al. 2018; Sosa-Gómez et al. 2020).
Evaluation of insecticide efficacy should not only measure mortality in pest populations within a given time period at lethal concentrations (Desneux et al. 2007; Stark and Banks 2003), but should also include examination of sublethal effects. The high mobility of stink bugs can limit their exposure to lethal concentrations, but these insecticides may still have important sublethal impacts (Lee et al. 2014; Morrison et al. 2017). Furthermore, many active components of chemicals can be influenced by factors such as temperature, rainfall, UV light or plant metabolism (Burrows et al. 2002; Hulbert et al. 2011; Maia et al. 2016), resulting in a degradation of the insecticide into low or sublethal concentrations. Measuring sublethal effects in addition to lethal effects of insecticides is therefore viewed as a more accurate assessment of insecticide efficacy (Desneux et al. 2007; Haynes 1988; Müller 2018; Stark and Banks 2003).
Sublethal concentrations of insecticides can affect many physiological parameters such as development, longevity, and fecundity (Zhou et al. 2017; Lu et al. 2020; Wu et al. 2022), as well as mobility (da Silva et al. 2022), and feeding behaviour (Koo et al. 2015; Zeng et al. 2016). These effects can vary according to the species and the insecticides used. For example, sublethal concentrations of dinotefuran can reduce the fecundity of the planthopper Nilaparvata lugens (Stal) (Bao et al. 2009) whereas no effect was observed on the mirid bug Apolygus lucorum (Meyer-Dur) (Lu et al. 2020). In contrast, other studies have shown that sublethal concentrations of insecticides can stimulate pest population dynamics (e.g., insecticide-induced hormetic responses) by influencing their reproductive output. For example, the insecticides triazophos and deltamethrin stimulate the reproductive systems of the adult males of N. lugens, leading to increased fertility in females after mating (Wang et al. 2010). A similar effect has been observed with Laodelphax striatellus (Fallen) where the fecundity and reproductive rate of females were increased after exposure to a sublethal dose of sulfoxaflor (Xu et al. 2016). On aphids, sublethal concentrations of beta-cypermethrin had no significant effect on Aulacorthum solani (Kaltenbach) whereas it can stimulate the oviposition of Aphis glycines (Matsumura) (Qu et al. 2020). Acceleration of development after exposure to sublethal concentrations of insecticides has also been reported in various species such as Bemisia tabaci (Gennadius) (Fang et al. 2018), Aphis gossypii (Glover) (Koo et al. 2015; Yuan et al. 2017) and N. lugens (Xu et al. 2022).
To date, research on the effects of insecticides on stink bugs has mainly focused on direct mortality (Sosa-Gómez et al. 2020) with few studies characterising sublethal effects. Cira et al. (2017) measured the sublethal effects of various insecticides on development and feeding of Halyomorpha halys (Stal), whereas Morrison et al. (2017) investigated the consequence of brief exposure on the survivorship and mobility of this pest. Hormetic effects have also been reported in Euschistus heros (F.) after exposure to sublethal concentrations of imidacloprid (Haddi et al. 2016; Santos et al. 2016).
The two-spotted stink bug, Bathycoelia distincta (Distant) is one of the major pests of macadamia in South Africa (Schoeman, 2013, 2018; Sonnekus et al. 2022). The damage from B. distincta is caused by the insertion of its stylet into the nuts for feeding, which can result in premature nut abscission and yield loss (Bruwer et al. 2021; Schoeman 2020). Stink bug feeding can also result in the deformation of nuts and development of lesions on the kernel, which decrease the overall quality of the nuts (Bruwer et al. 2021; Schoeman 2020). The use of pyrethroids remains the primary strategy for controlling B. distincta despite considerable effort in the implementation of integrated pest management methods in macadamia orchards in South Africa (Schoeman 2014a). Furthermore, macadamia trees can be very tall with dense branches which reduces the efficacy of insecticide applications (Schoeman 2014b).
To date, no experimental study has been conducted on the lethal and sublethal effects of insecticides on B. distincta. Here, we first evaluated the toxicity and effects on the mobility of B. distincta with four plant protection products frequently used in macadamia orchards, the insecticides acephate, lambda-cyhalothrin, beta-cyfluthrin and tau-fluvalinate. We then focused on lambda-cyhalothrin, the most commonly applied insecticide in macadamia orchards, and assessed its acute toxicity on adult B. distincta. Finally, the sublethal effects of lambda-cyhalothrin on biological parameters of the parent B. distincta (F0) and offspring generation (F1) were determined with the LC10 and LC30. Our objective was to determine whether the population-level performance and behavioural traits of B. distincta were influenced by insecticide exposure. The results from this study will be useful as a baseline reference point for future studies and may lead to improved stink bug management strategies for macadamia growers in South Africa.
METHODS
Insects
To test the various commercial products on B. distincta, egg masses of B. distincta were collected from a macadamia orchard in Limpopo (23°05'34.6" S, 30°14'23.3" E; South Africa), from February to April 2022 to start a laboratory colony at the Forestry and Agricultural Biotechnology Institute (FABI), Biological Control Centre of the University of Pretoria. Eggs were kept in small containers until hatching and bugs were subsequently transferred to a larger plastic container (27 × 15 cm) with a mesh lid for ventilation (approximatively 25 bugs per container). The stink bugs used to assess the sublethal effects of the pyrethroid lambda-cyhalothrin were initially collected in 2018 from a macadamia orchard in Limpopo (23°03'13" S, 30°14'02" E). The colony was maintained without any insecticide exposure since collection. All the insects used in this experiment were maintained in a climate-controlled chamber (25 ± 2 °C, 20 ± 5% RH, 14L:10D) and reared on green beans and corn ad libitum.
Time-mortality bioassays under manufacturers recommended application rate
Acute (lethal) toxicity was evaluated by direct contact of insecticides to the insect body of B. distincta via a glass-vial method (De Castro et al. 2018; Snodgrass et al. 2005). The bioassay was conducted in 20 ml glass vials treated with 0.5 ml solution of each insecticide. All the insecticides used are registered for controlling B. distincta in macadamia orchards (SAMAC 2022). The insecticides used and their respective commercial formulations were: the organophosphate acephate (Ace' 750SP; 750 g a.i.kg-1; Nulandis), and the pyrethroids lambda-cyhalothrin (Karate Zeon' 10 CS; 100 g a.i.l-1; Syngenta South Africa Ltd), beta-cyfluthrin (Buldock' Beta 125 SC; 125 g a.i.l-1; Bayer (Pty) Ltd) and tau-fluvalinate (Klartan® 240 EW; 240 g a.i.l-1; Adama South Africa (Pty) Ltd). The commercial formulations were diluted in 1 l of distilled water, achieving the following concentration for the bioassays: 75 mg l-1 (acephate), 5 mg l-1 (lambda-cyhalothrin), 7.5 mg l-1 (beta-cyfluthrin) and 72 mg l-1 (tau-fluvalinate). The vials were rolled on a hotdog roller with the heat unit disconnected in a fume hood until the commercial formulations diluted with water had evaporated and the insecticide covered the inner vial surface. For the control treatment, vials were treated with distilled water only. Insects were placed individually into treated vials plugged with cotton to prevent escape with no food. Preliminary tests confirmed that B. distincta can survive without food or water for time periods up to 48 h in the glass vials.
Twenty B. distincta adults (sex ratio 1:1) were evaluated individually for response to each insecticide treatment. The insects were kept in a climate-controlled chamber (25 ± 2 °C, 20 ± 5% RH, L14:D10) and exposure was replicated three times. Insect mortality was observed every 2 h during the initial 12 h exposure and at 6 h intervals afterwards until death. Bugs were recorded as dead if they did not move when probed with a paintbrush.
Concentration-mortality bioassays
Lambda-cyhalothrin toxicity to B. distincta was determined via the same method described above. A gradient of concentration of lambda-cyhalothrin (99% purity, Sigma-Aldrich, Johannesburg, South Africa) was prepared and diluted in acetone (Sigma-Aldrich, Johannesburg, South Africa). Insects were placed individually into treated vials plugged with cotton to prevent escape with no food.
Three replicates of seven concentrations (10 μg/l, 30 μg/l, 75 μg/l, 100 μg/l, 300 μg/l, 750 μg/l, 1000 μg/l), and a control without insecticide were tested, and ten stink bugs per concentration were used (sex ratio 1:1, n = 240). The concentrations used were established through preliminary bioassays to facilitate identification of the concentration range that leads to mortality > 0 (between 0 and 50) and < 100 (between 90 and 100). All the vials were maintained in a climate-controlled chamber (25 ± 2 °C, 20 ± 5% RH, L14:D10). Mortality was assessed after 24 and 48 h of exposure. Insects were considered dead if they did not move when probed with a paintbrush.
Behavioural bioassays
Horizontal movement
Bathycoelia distincta movement was analysed using a Petri-dish method (Leskey et al. 2012; Morrison et al. 2017). Adults were briefly exposed to commercial formulation of insecticides in glass-treated vials for 10 min and subsequently transferred into an untreated glass Petri dish arena (90 mm diameter x 15 mm high) with a lid to prevent escape. Ten insects (sex ratio 1:1) per insecticide were tested per treatment in a complete randomised experimental design. Petri dishes were washed after each use with soap and water and dried in an oven overnight at 110 °C.
The horizontal movement of adults was tracked for 10 min immediately after insecticide exposure with a Logitech© C922 Pro HD stream webcam, suspended approximatively 20 cm directly above the centre of the arena. All bioassays were conducted in a custom-made white box (90 x 100 x 100 cm) in a climate-controlled chamber maintained at 25 ± 2 °C and 20 ± 5% relative humidity. The software EthoVision© XT (version 16) was used to analyse the videos and to measure the distance travelled (cm) and angular velocity (degrees/sec) of the insects in the arena.
Sublethal effects of lambda-cyhalothrin on life-history traits of B. distincta
Fifth instar stink bugs were placed on green beans and corn until they moulted to adults. These adults were then used in the study as the F0 generation individuals to ensure that all stink bugs were similar in age prior to exposure to insecticide. The LC10 and LC30 obtained from the experiment above were used to evaluate the sublethal effect of lambda-cyhalothrin on B. distincta. The LC10 and LC30 were chosen to mimic lower concentrations of lambda-cyhalothrin that may occur in the field following initial insecticide application owing to its degradation by various factors. The two concentrations were prepared in acetone following the method described above, and acetone was used as a control. Adults were exposed via treated glass-vials. After 48 h, male and female survivors of each treatment were paired (1 male and 1 female, 6 pairs per treatment) and transferred to small plastic containers (11 × 11 × 15 cm) with food. Every 2 days, adult mortality and fecundity were recorded. The number of eggs were counted until female adult death. The hatched eggs were recorded, and the nymphs were transferred into small plastic containers. Nymphal stage and survival were checked and recorded every 2 days. Finally, when the nymphs reached the adult stage, the sex ratio was assessed for each treatment. All the experiments were conducted in a climate-controlled chamber (25 ± 2 °C, 20 ± 5% RH, 14 L:10 D) and insects were fed ad libitum with green beans and corn.
Statistical analyses
The time-mortality data were subjected to survival analysis using the packages survival and survminer (Kassambara and Kosinski 2018; Therneau 2015), and the Kaplan-Meier method was used followed by a post-hoc test for comparison of survival between treatments. Concentration-mortality bioassays were subjected to Probit analysis (Finney 1971; Robertson et al. 2007; Wheeler et al. 2006) using the ecotox package in R (Hlina 2020). Concentration was log-transformed to calculate the lethal concentration for 10%, 30% and 50% (LC10, LC30 and LC50) of the population after 48 h, as well as their 95% confidence limits (CI). No stink bug mortality occurred in the control treatment in the toxicity bioassays, so adjustment of treatment mortalities was not required (Abbott 1925). The data from the behavioural bioassays (distance walked, angular velocity) and the sublethal bioassays were tested for normality of residuals and homogeneity of variance using a Kolmogorov-Smirnov and Bartlett's test, respectively. When these assumptions were met (inspection of residuals and by Bartlett's test), one-way ANOVA was performed, and the means were compared using Tukey's honestly significant difference (HSD) test. If the assumptions were violated a Kruskal-Wallis test was performed followed by a Dunn post-hoc test to compare the differences between treatments using the package FSA (Ogle et al. 2023). Only the adult longevity data were analysed with a two-way ANOVA (factors: sex and treatment). All data were analysed using R (version 4.1.2) and significance was accepted at α = 0.05.
RESULTS
Time-mortality bioassays
The survival analysis of B. distincta exposed to dried insecticide residues indicated significant differences among treatments (Log-rank test, χ2 = 117, df = 4, p < 0.001) (Figure 1). No mortality of B. distincta was observed in the control (without insecticide exposure) after 72 h of exposure, while 100% mortality was observed for the insecticides acephate, beta-cyfluthrin, lambda-cyhalothrin and tau-fluvalinate after 36, 48, and 72 h, respectively. Such differences were reflected in the mean survival time (LT50) observed for each insecticide. The LT50 were 8 h and 30 h for the insecticides acephate and beta-cyfluthrin, and 36 h for the insecticides lambda-cyhalothrin and tau-fluvalinate. The mean survival time was not estimated for stink bugs without exposure (control) because no mortality was observed.
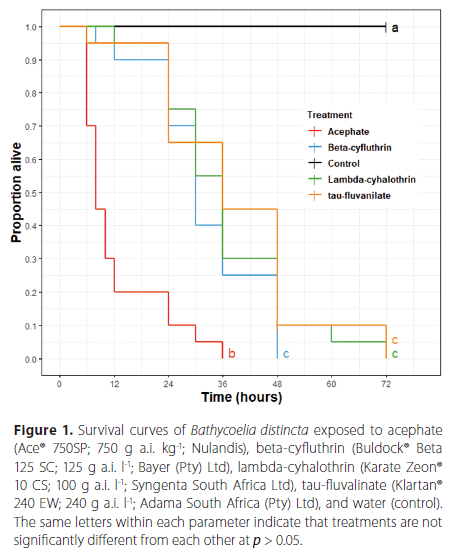
Concentration-mortality bioassays
Standard probit analysis of concentration-mortality data showed that after 48 h of exposure to lambda-cyhalothrin, the LC50 value was estimated at 157 μg/ml (Table 1). The LC10 and LC30 values were estimated at 22.8 and 71.2 μg/ml, respectively, and were used for the subsequent assessment of sublethal effects.
Horizontal movement
Effects of exposure to insecticide residues were observed on the distance walked immediately after insecticide exposure (Figure 2A, χ2 = 29.70, df = 4, p < 0.001) and post-hoc analyses suggest different trends between treatments. After 10 min of insecticide exposure, B. distincta adults moved significantly greater distance for the three insecticides beta-cyfluthrin, lambda-cyhalothrin and tau-fluvalinate than acephate or the control (Dunn's test, p < 0.05). The distance walked by adults exposed to acephate for 10 min was not significantly different from the control (Dunn's test, p > 0.05). Effects of exposure to insecticide residues were also observed on the angular velocity (Figure 2B, F = 10.48, df = 4, p < 0.001). The angular velocity significantly decreased for the pyrethroids beta-cyfluthrin, lambda-cyhalothrin and tau-fluvalinate compared to acephate or the control (Tukey HSD, p < 0.05). The angular velocity between adults exposed to acephate was not significantly different from the control (Tukey HSD, p > 0.05).
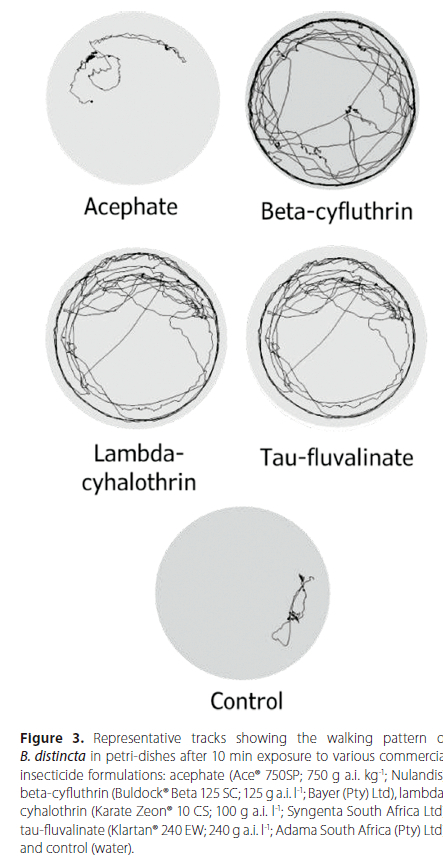
Video recordings showed that movement patterns consisted mainly of circular paths along the arena edges, which were different from those observed in acephate or control adults (Figure 3).
Sublethal effects of lambda-cyhalothrin on the F0 generation
Adult B. distincta longevity was significantly affected by treatments (F = 10.77, df = 2, p < 0.001) and sex (F = 9.04, df = 1, p < 0.001) (Figure 4A). LC30 significantly reduced adult male longevity when compared with the LC10 and control treatments (Figure 4A, Tukey's HSD tests, α = 0.05). Exposure to sublethal concentrations of lambda-cyhalothrin significantly affected the fecundity of B. distincta (Figure 4B, F = 8.55, df = 2, p < 0.001). The number of eggs produced per female was significantly lower when stink bugs were exposed to LC30 (40 ± 24 eggs laid) compared to control treatments (170 ± 28 eggs laid) (Tukey HSD, α < 0.05). No significant difference was observed in the fecundity between the LC10 and LC30 or control treatment (Tukey HSD, α > 0.05).
Sublethal effects of lambda-cyhalothrin on the F1 generation
The development duration, hatching rate, survival of nymphs and sex ratio of the F1 generation of B. distincta are shown in Figure 5. Both LC10 and LC30 had no significant effect on the hatching rate of eggs (Figure 5A, χ2 = 1.93, df = 2, p > 0.05). Sublethal concentrations of lambda-cyhalothrin had significant effects on the total development time from egg to the adult stage (Figure 5B, χ2 = 31.64, df = 2, p < 0.001). The total developmental time from egg to the adult stage was significantly shorter for LC10 and LC30 compared to the control treatment (Dunn's test p < 0.05). The LC10 and LC30 significantly decreased the developmental duration time at the egg stage χ2 = 22.67, df = 2, p < 0.001) and of the 4th = 23.64, df = 2,p < 0.001) and 5th instar (χ2 = 1.514, df = 2, p < 0.01) compared to the control treatment (Figure 5C). In comparison, the LC30 significantly increased the development time of the 3rd instar compared to the control, whereas the LC10 significantly decreased it compared to the control (χ2 = 24.50, df = 2, p < 0.001). Nymphal survival was lower during the 2nd instar when stink bugs were exposed to LC10 compared to the control treatment (Figure 5D, χ2 = 6.02, df = 2, p < 0.05). No significant difference was observed in the sex ratio of the F1 generation (determined at the adult stage) between the various treatments (Figure 5E, F = 0.59, df = 2, p > 0.05).
DISCUSSION
This study evaluated the toxicity and behavioural effects of four commercial insecticides used to control the stink bug pest B. distincta in macadamia orchards. The bioassays revealed that the organophosphate acephate was more toxic (LT50 = 8 h) than the pyrethroids lambda-cyhalothrin (LT50 = 36 h), beta-cyfluthrin (LT50 = 30 h) and tau-fluvalinate (LT50 = 36 h) under insecticide field rates. In the behavioural bioassays, the pyrethroids induced an increase in the distance walked and a decrease of angular velocity, suggesting that commercial application of pyrethroids might result in a higher dispersal and low mortality. The effects of sublethal concentrations (LC10 and LC30) of the active ingredient of lambda-cyhalothrin were subsequently assessed on the F0 and F1 generation of B. distincta. Knowledge regarding the sublethal effects of insecticides is relevant for insect pest management since target species are often exposed to sublethal concentrations of these compounds for longer periods than lethal concentrations due to insecticide degradation. In the F0 generation, the LC30 significantly reduced male longevity and female fecundity. For the F1 generation, sublethal concentrations (LC10 and LC30) of lambda-cyhalothrin significantly accelerated the egg stage, and the 3rd, 4th and 5th instar developmental time compared to the control, resulting in a shorter developmental time.
In our study, toxicity bioassays revealed that B. distincta is more tolerant to pyrethroids than organophosphates. The toxicity of pyrethroids and organophosphate insecticides to the Pentatomidae can be variable among species (Blackman et al. 2015; Cira et al. 2017; Lee, Short, et al. 2014; Leskey et al. 2012; Nielsen et al. 2008; Pazini et al. 2019; Snodgrass et al. 2005; Tillman and Mullinix 2004). For example, H. halys was able to recover after exposure to various pyrethroids (Leskey et al. 2012; Nielsen et al. 2008), whereas Tillman and Mullinix (2004) showed that E. servus and P. maculiventris from Georgia were not able to recover from pyrethroid exposure. In the southeastern United States, Euschistus servus was more tolerant to pyrethroid and organophosphate insecticide exposures compared to Chinavia hilaris (Say), and Nezara viridula (L) (Snodgrass et al. 2005). Therefore, the difference in tolerance observed in this study could be a B. distincta specific trait, and other species associated with macadamia orchards might respond differently.
The higher tolerance of B. distincta to the commercial pyrethroids than the organophosphate could also be linked to management practices since the egg masses were collected from commercial macadamia orchards. For example, intensive and repeated applications of acephate and lambda-cyhalothrin linked to the expansion of soybean production in Brazil, reduced the susceptibility of E. heros to these insecticides considerably (Sosa-Gómez et al. 2020; Somavilla et al. 2020; Tuelher et al. 2018). The recommended doses of acephate increased four-fold in 14 years (Sosa-Gómez et al. 2020). Similarly, the rapid expansion of macadamia-planted areas in South Africa has led to the increase of insecticide applications, especially pyrethroids, and a decline in the susceptibility in B. distincta populations has been suggested (Schoeman 2014a). Although this study constitutes the first toxicity assays conducted on B. distincta, future research should also evaluate individuals from populations with variable exposure histories.
Changes in locomotory behaviour of B. distincta were observed after brief exposure to commercial insecticide formulations. These effects are not surprising since neurotoxic insecticides were used which can trigger distinct behavioural responses (Desneux et al. 2007). In our study, sublethal doses of the pyrethroids increased the distance walked and decreased the angular velocity of B. distincta movements, while no differences were observed between bugs exposed to organophosphate and bugs without exposure. Lee et al. (2013) obtained similar results where pyrethroid insecticides caused immediate neurotoxicity on H. halys that resulted in rapid and uncoordinated movement compared to organophosphates where the effects were slower. Alterations in walking behaviour has been shown to be a strategy to overcome the action of insecticides (Haddi et al. 2015; Morales et al. 2013) and it is possible that surviving B. distincta could disperse from insecticide-contaminated areas, since stink bugs are highly mobile (Lee et al. 2014). Thus, further studies should test behavioural responses over longer time periods (i.e., a few hours) and test whether B. distincta can leave areas treated with pyrethroid and organophosphate residues.
In this study, the two sublethal concentrations (LC10 and LC30) of lambda-cyhalothrin did not impact female longevity whereas the LC30 decreased male longevity significantly. Similar results have been observed in the mirid bug A. lucorum, where the authors suggested that the susceptibility to insecticides may be correlated to size and weight (Tan et al. 2012). However, Nielsen et al. (2008) tested several insecticides and observed that H. halys males were less susceptible to thiamethoxam than females despite having smaller body mass. It is therefore unclear if body mass could explain the decreased male B. distincta longevity after exposure to lambda-cyhalothrin observed in this study. Other parameters such as detoxifying enzymes or target-site insensitivity may be involved. Previous studies have shown that detoxification of insecticides in pentatomids is associated with P450 monooxygenases, glutathione-S-transferases, α-esterase, and β-esterase enzymes (Bansal and Michel 2018; Boff et al. 2022; Mittapelly et al. 2019; Sosa-Gómez et al. 2009; Sosa-Gómez et al. 2020; Sparks et al. 2020), and differential expression of P450s genes among sexes can occur (Huber et al. 2007; Musasia et al. 2013; Zuo and Chen 2014). Thus, detoxification mechanisms might also occur in B. distincta and enzyme expression levels should be investigated in the future.
The fecundity of females exposed to LC30 of lambda-cyhalothrin was significantly reduced compared to the control. Several studies have demonstrated effects of sublethal concentrations of insecticides on fecundity (Bao et al. 2009; Wu et al. 2022) where females exposed can exhibit compensatory effects, resulting in a higher reproductive performance and a shorter life span (Vilca Mallqui et al. 2014; Santos et al. 2016). This has been demonstrated in female E. heros with sublethal concentrations of imidacloprid which increased their fecundity and fertility rates but reduced their longevity (Santos et al. 2016). In addition, another study showed that sublethal exposure of imidacloprid can increase the mating frequency of males and induced higher fecundity rates (Haddi et al. 2016). In our study no effect was observed on the hatching rate of eggs from exposed and nonexposed females. Further studies are required to determine if female B. distincta exposed to LC30 were mated or not. Female and male reproductive organs and their morphology (e.g., testicles, sperm mobility, sperm storage, ovaries, ovarian cells, etc.) should also be investigated in the future to determine the effects of sublethal exposure of lambda-cyhalothrin on the reproductive capacity of B. distincta, in order to explain the significant differences in fertility observed in this study.
Sublethal exposure of the parent generation (F0) to lambda-cyhalothrin stimulated F1 nymphal development in B. distincta, resulting in a shorter total developmental time to the adult stage and low overall mortality. Acceleration of development has been documented and the effect seems dependent on the insecticide, as well as the pest. For example, sublethal concentrations of imidacloprid shortened nymphal development in A. gossypii (Koo et al. 2015; Yuan et al. 2017) and A. glycines (Qu et al. 2015) whereas in other species such as Rhopalosiphum padi (L.) (Li et al. 2018) or Myzus persicae (Sulzer) (Zeng et al. 2016), the duration of nymphal development was extended. Our results suggest that a low dose of lambda-cyhalothrin on B. distincta can accelerate development in the F1 generation which could increase the population size of this pest and likely cause resurgence and outbreaks in the field (Cordeiro et al. 2013; Guedes and Cutler 2014) which may explain the decline of susceptibility in B. distincta populations observed previously (Schoeman 2014a).
In this study, various commercial insecticide formulations were tested on B. distincta adults and the sublethal effects of the active ingredient lambda-cyhalothrin were investigated in the F0 and F1 generation of B. distincta. Brief exposure to pyrethroids at field application rates stimulated the walking behaviour of B. distincta whereas at sublethal concentrations the pyrethroid lambda-cyhalothrin accelerated the development of offspring. Our results suggest that it is important to consider changes in mobility and effects that sublethal concentrations of insecticide could have on the development and reproduction of the target pest. These behavioural changes should be taken into consideration for IPM programmes in macadamia orchards.
Future experiments should be expanded to greenhouse and field conditions to confirm these results and to determine the mechanism of action of pyrethroids on B. distincta.
ACKNOWLEDGEMENTS
This research was funded by the University of Pretoria, the Forestry and Agricultural Biotechnology Institute (FABI) and the African Centre of Chemical Ecology at the University of Pretoria, the DSI-NRF Centre of Excellence in Plant Health Biotechnology (CPHB grant number 40945), Macadamia South Africa NPC (SAMAC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest was reported by the authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Elisa Pal: Conceptualisation; investigation; methodology; data curation; formal analysis; project administration; writing-original draft; writing-review and editing.
Jeremy D. Allison: supervision; writing - review and editing.
Brett P. Hurley: supervision; writing - review and editing.
Bernard Slippers: supervision; writing - review and editing.
Gerda Fourie: funding acquisition; supervision; writing - review and editing.
ORCID IDS
Elisa Pal: https://orcid.org/0000-0002-5799-3440
Jeremy D. Allison: https://orcid.org/0000-0002-0765-3149
Brett P. Hurley: https://orcid.org/0000-0002-8702-5547
Bernard Slippers: https://orcid.org/0000-0003-1491-3858
Gerda Fourie: https://orcid.org/0000-0003-2650-5448
REFERENCES
Abbott W. 1925. A method of computing the effectiveness of an insecticide Journal of Economic Entomology. 18(2):265-267. https://doi.org/10.1093/jee/18.2.265a. [ Links ]
Bansal R, Michel A. 2018. Expansion of cytochrome P450 and cathepsin genes in the generalist herbivore brown marmorated stink bug. BMC Genomics. 19(1):60. https://doi.org/10.1186/s12864-017-4281-6. [ Links ]
Bao H, Liu S, Gu J, Wang X, Liang X, Liu Z. 2009. Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Management Science. 65(2):170-174. https://doi.org/10.1002/ps.1664. [ Links ]
Blackman B, Lanka S, Hummel N, Way M, Stout M. 2015. Comparison of the effects of neonicotinoids and pyrethroids against Oebalus pugnax (Hemiptera: Pentatomidae) in rice. Florida Entomologist. 98(1):18-26. https://doi.org/10.1653/024.098.0104. [ Links ]
Boff JS, Reis AC, Patricia DSG, Pretto VE, Garlet CG, Melo AA, Bernardi O. 2022. The effect of synergistic compounds on the susceptibility of Euschistus heros (Hemiptera: Pentatomidae) and Chrysodeixis includens (Lepidoptera: Noctuidae) to pyrethroids. Environmental Entomology. 51(2):421-429. https://doi.org/10.1093/ee/nvac005. [ Links ]
Bruwer IJ, Giliomee JH, Pringle KL. 2021. The relationship between proboscis length and the ability of certain Heteroptera to damage macadamia kernels. African Entomology. 29(1):112-124. https://doi.org/10.4001/003.029.0112. [ Links ]
Burrows HD, Canle LM, Santaballa JA, Steenken S. 2002. Reaction pathways and mechanisms of photodegradation of pesticides. Journal of Photochemistry and Photobiology B. 67(2):71-108. https://doi.org/10.1016/S1011-1344(02)00277-4. [ Links ]
Cira TM, Burkness EC, Koch RL, Hutchison WD. 2017. Halyomorpha halys mortality and sublethal feeding effects following insecticide exposure. Journal of Pest Science. 90(4):1257-1268. https://doi.org/10.1007/s10340-017-0871-y. [ Links ]
Cordeiro EMG, de Moura ILT, Fadini MAM, Guedes RNC. 2013. Beyond selectivity: are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere. 93(6):1111-1116. https://doi.org/10.1016/jxhemosphere.2013.06.030. [ Links ]
da Silva WR, Pereira RC, Mendonça LVP, Peçanha LS, de Sales Abreu LM, Abib PHN, Samuels RI, Picanço MC, Silva GA. 2022. Lethal and sublethal effects of insecticides used in the management of Plutella xylostella (Lepidoptera: Plutellidae) on the predator Cycloneda sanguinea L. (Coleoptera: Coccinellidae). Pest Management Science. 78(10):4397-4406. https://doi.org/https://doi.org/10.1002/ps.7060. [ Links ]
de Castro AA, Legaspi JC, Tavares WDS, Meagher RL, Miller N, Kanga L, Haseeb M, Serräo JE, Wilcken CF, Zanuncio JC. 2018. Lethal and behavioral effects of synthetic and organic insecticides on Spodoptera exigua and its predator Podisus maculiventris. PLoS One. 13(11):e0206789. https://doi.org/10.1371/journal.pone.0206789. [ Links ]
Desneux N, Decourtye A, Delpuech J-M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology. 52(1):81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440. [ Links ]
Finney DJ. 1971. Probit Analysis (3rd Edition ed.). Cambridge University Press. https://onlinelibrary.wiley.com/doi/abs/10.1002/jps.3030411125
Greene JK, Baum JA, Benson EP, Bundy CS, Jones WA, Kennedy GG, McPherson JE, Musser FR, Reay-Jones FPF, Toews MD, et al. 2018. General insect management. In: McPherson JE, editor. Invasive Stink Bugs and Related Species (Pentatomoidea). CRC Press. pp 729-774. https://doi.org/10.1201/9781315371221-16.
Guedes RNC, Cutler GC. 2014. Insecticide-induced hormesis and arthropod pest management. Pest Management Science. 70(5):690-697. https://doi.org/10.1002/ps.3669. [ Links ]
Haddi K, Mendes MV, Barcellos MS, Lino-Neto J, Freitas HL, Guedes RNC, Oliveira EE. 2016. Sexual success after stress? Imidacloprid-induced hormesis in males of the Neotropical stink bug Euschistus heros. PLoS One. 11(6):e0156616. https://doi.org/10.1371/journal.pone.0156616. [ Links ]
Haddi K, Mendonça LP, Dos Santos MF, Guedes RNC, Oliveira EE. 2015. Metabolic and behavioral mechanisms of indoxacarb resistance in Sitophilus zeamais (Coleoptera: curculionidae). Journal of Economic Entomology. 108(1):362-369. https://doi.org/10.1093/jee/tou049. [ Links ]
Haynes KF. 1988. Sublethal effects of neurotoxic insecticides on insect behavior. Annual Review of Entomology. 33(1):149-168. https://doi.org/10.1146/annurev.en.33.010188.001053. [ Links ]
Hlina BL . 2020. ecotox: A nalysis of ecotoxicology. R package version 1.4.2.
Huber DPW, Erickson ML, Leutenegger CM, Bohlmann J, Seybold SJ. 2007. Isolation and extreme sex-specific expression of cytochrome P450 genes in the bark beetle, Ips paraconfusus, following feeding on the phloem of host ponderosa pine, Pinus ponderosa. Insect Molecular Biology. 16(3):335-349. https://doi.org/10.1111/j.1365-2583.2007.00731.x. [ Links ]
Hulbert D, Isaacs R, Vandervoort C, Wise JC. 2011. Rainfastness and residual activity of insecticides to control Japanese beetle (Coleoptera: Scarabaeidae) in grapes. Journal of Economic Entomology. 104(5):1656-1664. https://doi.org/10.1603/EC11077. [ Links ]
Kassambara A, Kosinski M. 2018. "Survminer": Drawing Survival Curves using "ggplot2". R package version 0.4.2. https://CRAN.R-project.org/package=survminer
Koo H-N, Lee S-W, Yun S-H, Kim HK, Kim G-H. 2015. Feeding response of the cotton aphid, Aphis gossypii, to sublethal rates of flonicamid and imidacloprid. Entomologia Experimentalis et Applicata. 154(2):110-119. https://doi.org/10.1111/eea.12260. [ Links ]
Lee D-H, Nielsen AL, Leskey TC. 2014. Dispersal capacity and behavior of nymphal stages of Halyomorpha halys (Hemiptera: Pentatomidae) evaluated under laboratory and field conditions. Journal of Insect Behaviour. 27(5):639-651. https://doi.org/10.1007/s10905-014-9456-2. [ Links ]
Lee D-H, Short BD, Nielsen AL, Leskey TC. 2014. Impact of organic insecticides on the survivorship and mobility of Halyomorpha halys (Stâl) (Hemiptera: Pentatomidae) in the laboratory. Florida Entomologist. 97(2):414-421. https://doi.org/10.1653/024.097.0211. [ Links ]
Leskey TC, Lee D-H, Short BD, Wright SE. 2012. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. Journal of Economic Entomology. 105(5):1726-1735. https://doi.org/10.1603/EC12096. [ Links ]
Li W, Lu Z, Li L, Yu Y, Dong S, Men X, Ye B. 2018. Sublethal effects of imidacloprid on the performance of the bird cherry-oat aphid Rhopalosiphum padi. PLoS One. 13(9):e0204097. https://doi.org/10.1371/journal.pone.0204097. [ Links ]
Lu Z, Dong S, Li C, Li L, Yu Y, Men X, Yin S. 2020. Sublethal and transgenerational effects of dinotefuran on biological parameters and behavioural traits of the mirid bug Apolygus lucorum. Scientific Reports. 10(1):226-234. https://doi.org/10.1038/s41598-019-57098-z. [ Links ]
Maia JB, Carvalho GA, Medina P, Garzón A, Gontijo PDC, Viñuela E. 2016. Lethal and sublethal effects of pesticides on Chrysoperla carnea larvae (Neuroptera: Chrysopidae) and the influence of rainfastness in their degradation pattern over time. Ecotoxicology. 25(5):845-855. https://doi.org/10.1007/s10646-016-1641-y. [ Links ]
McPherson JE (ed). 2018. Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management. Oxford University Press. https://doi.org/10.1201/9781315371221.
McPherson JE, McPherson RM. 2000. Stink bugs of economic importance in America North of Mexico. CRC Press. https://doi.org/10.1201/9781420042429.
Mittapelly P, Bansal R, Michel A. 2019. Differential expression of cytochrome P450 CYP6 genes in the brown marmorated stink bug, Halyomorpha halys (Hemiptera: pentatomidae). Journal of Economic Entomology. 112(3):1403-1410. https://doi.org/10.1093/jee/toz007. [ Links ]
Morales JA, Cardoso DG, Della Lucia TMC, Guedes RNC. 2013. Weevil x insecticide: Does 'personality' matter? PLoS One. 8(6):e67283. https://doi.org/10.1371/journal.pone.0067283. [ Links ]
Morrison WR 3rd, Poling B, Leskey TC. 2017. The consequences of sublethal exposure to insecticide on the survivorship and mobility of Halyomorpha halys (Hemiptera: pentatomidae). Pest Management Science. 73(2):389-396. https://doi.org/10.1002/ps.4322. [ Links ]
Müller C. 2018. Impacts of sublethal insecticide exposure on insects - facts and knowledge gaps. Basic and Applied Ecology. 30:1-10. https://doi.org/10.1016/j.baae.2018.05.001. [ Links ]
Musasia FK, Isaac AO, Masiga DK, Omedo IA, Mwakubambanya R, Ochieng R, Mireji PO. 2013. Sex-specific induction of CYP6 cytochrome P450 genes in cadmium and lead tolerant Anopheles gambiae. Malaria Journal. 12(1):97. https://doi.org/10.1186/1475-2875-12-97. [ Links ]
Nielsen AL, Shearer PW, Hamilton GC. 2008. Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. Journal of Economic Entomology. 101(4):1439-1442. https://doi.org/10.1603/0022-0493(2008)101. [ Links ]
Ogle DH, Doll JC, Wheeler AP, Dinno A. 2023. FSA: Simple Fisheries Stock Assessment Methods. R package version 0.9.4. https://fishr-core-team.github.io/FSA/.
Pazini JB, Padilha AC, Cagliari D, Bueno FA, Rakes M, Zotti MJ, Martins JFS, Grützmacher AD. 2019. Differential impacts of pesticides on Euschistus heros (Hem.: Pentatomidae) and its parasitoid Telenomus podisi (Hym.: Platygastridae). Scientific Reports. 9(1):6544. https://doi.org/10.1038/s41598-019-42975-4. [ Links ]
Qu Y, Ullah F, Luo C, Monticelli LS, Lavoir A-V, Gao X, Song D, Desneux N. 2020. Sublethal effects of beta-cypermethrin modulate interspecific interactions between specialist and generalist aphid species on soybean. Ecotoxicology and Environmental Safety. 206:111302. https://doi.org/10.1016/j.ecoenv.2020.111302. [ Links ]
Qu Y, Xiao D, Li J, Chen Z, Biondi A, Desneux N, Gao X, Song D. 2015. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology. 24(3):479-487. https://doi.org/10.1007/s10646-014-1396-2. [ Links ]
Robertson JL, Savin NE, Savin NE, Preisler HK. 2007. Bioassays with Arthropods, 2nd edition. Boca Raton: CRC Press. https://doi.org/10.1201/9781420004045. [ Links ]
SAMAC. 2022. Registered products and MRLs. [accessed 2022, Jan 10]. https://samac.org.za/registered-products. Registration required.
Santos MF, Santos RL, Tomé HVV, Barbosa WF, Martins GF, Guedes RNC, Oliveira EE. 2016. Imidacloprid-mediated effects on survival and fertility of the Neotropical brown stink bug Euschistus heros. Journal of Pest Science. 89(1):231-240. https://doi.org/10.1007/s10340-015-0666-y. [ Links ]
Schoeman PS. 2013. Phytophagous stink bugs (Hemiptera: Pentatomidae; Coreidae) associated with macadamia in South Africa. Open Journal of Animal Sciences. 3(3):179-183. https://doi.org/10.4236/ojas.2013.33027. [ Links ]
Schoeman PS. 2014b. Aspects affecting distribution and dispersal of the indigenous Heteroptera complex (Heteroptera: Pentatomidae & Coreidae) in South African macadamia orchards. African Entomology. 22(1):191-196. https://doi.org/10.4001/003.022.0130. [ Links ]
Schoeman PS. 2014a. Stink bug IPM on macadamias in South Africa: current status and the road ahead. Trends in Entomology. 10:87-95. [ Links ]
Schoeman PS. 2018. Relative seasonal occurrence of economically significant Heteropterans (Pentatomidae and Coreidae) on macadamias in South Africa: implications for management. African Entomol. 26(2):543-549. https://doi.org/10.4001/003.026.0543. [ Links ]
Schoeman PS. 2020. Damage potential of indigenous Heteroptera species occurring on Macadamia nuts (Macadamia integrifolia Maiden & Betche & Macadamia tetraphylla L. Johnson) in South Africa during the early and late season. International Journal of Tropical Insect Science. 40(1):217-219. https://doi.org/10.1007/s42690-019-00041-6. [ Links ]
Snodgrass GL, Adamczyk JJ Jr, Gore J. 2005. Toxicity of insecticides in a glass-vial bioassay to adult brown, green, and Southern green stink bugs (Heteroptera: pentatomidae). Journal of Economic Entomology. 98(1):177-181. https://doi.org/10.1093/jee/98.L177. [ Links ]
Somavilla JC, Reis AC, Gubiani PDS, Godoy DN, Stürmer GR, Bernardi O. 2020. Susceptibility of Euschistus heros and Dichelops furcatus (Hemiptera: Pentatomidae) to selected insecticides in Brazil. Journal of Economic Entomology. 113(2):924-931. https://doi.org/10.1093/jee/toz340. [ Links ]
Sonnekus B, Slippers B, Hurley BP, Joubert E, Stiller M, Fourie G. 2022. Diversity and molecular barcoding of stink bugs (Hemiptera: Pentatomidae) associated with macadamia in South Africa. Insects. 13(7):601. https://doi.org/10.3390/insects13070601. [ Links ]
Sosa-Gómez DR, Da Silva JJ, De Oliveira Negrao Lopes I, Corso IC, Almeida AMR, Piubelli De Moraes GC, Baur ME. 2009. Insecticide susceptibility of Euschistus heros (Heteroptera: Pentatomidae) in Brazil. Journal of Economic Entomology. 102(3):1209-1216. https://doi.org/10.1603/029.102.0346. [ Links ]
Sosa-Gómez DR, Corrêa-Ferreira BS, Kraemer B, Pasini A, Husch PE, Delfino Vieira CE, Reis Martinez CB, Negräo Lopes IO. 2020. Prevalence, damage, management and insecticide resistance of stink bug populations (Hemiptera: Pentatomidae) in commodity crops. Agricultural and Forest Entomology. 22(2):99-118. https://doi.org/10.1111/afe.12366. [ Links ]
Sparks ME, Bansal R, Benoit JB, Blackburn MB, Chao H, Chen M, Cheng S, Childers C, Dinh H, Doddapaneni HV, et al. 2020. Brown marmorated stink bug, Halyomorpha halys (Stâl), genome: putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest. BMC Genomics. 21(1):227. https://doi.org/10.1186/s12864-020-6510-7. [ Links ]
Stark JD, Banks JE. 2003. Population-level effects of pesticides and other toxicants on arthropods. Annual Review of Entomology. 48(1):505-519. https://doi.org/10.1146/annurev.ento.48.091801.112621. [ Links ]
Tan Y, Biondi A, Desneux N, Gao X-W. 2012. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology. 21(7):1989-1997. https://doi.org/10.1007/s10646-012-0933-0. [ Links ]
Therneau T. 2015. "Survival": A Package for Survival Analysis in S. R package version 2.38. https://CRAN.R-project.org/package=survival
Tillman PG, Mullinix BG. 2004. Comparison of susceptibility of pest Euschistus servus and predator Podisus maculiventris (Heteroptera: Pentatomidae) to selected insecticides. Journal of Economic Entomology. 97(3):800-806. https://doi.org/10.1603/0022-0493(2004)097[0800:COSOPE]2.0.CO;2. [ Links ]
Tuelher ES, da Silva ÉH, Rodrigues HS, Hirose E, Guedes RNC, Oliveira EE. 2018. Area-wide spatial survey of the likelihood of insecticide control failure in the neotropical brown stink bug Euschistus heros. Journal of Pest Science. 91(2):849-859. https://doi.org/10.1007/s10340-017-0949-6. [ Links ]
Vilca Mallqui KS, Vieira JL, Guedes RNC, Gontijo LM. 2014. Azadirachtin-induced hormesis mediating shift in fecundity-longevity trade-off in the Mexican bean weevil (Chrysomelidae: bruchinae). Journal of Economic Entomology. 107(2):860-866. https://doi.org/10.1603/EC13526. [ Links ]
Wang L-P, Shen J, Ge L-Q, Wu J-C, Yang G-Q, Jahn GC. 2010. Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stâl (Hemiptera: delphacidae). Crop Protection. 29(11):1280-1285. https://doi.org/10.1016/j.cropro.2010.07.009. [ Links ]
Wheeler MW, Park RM, Bailer AJ. 2006. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environmental Toxicology and Chemistry. 25(5):1441-1444. https://doi.org/10.1897/05-320R.1. [ Links ]
Wu H-M, Feng H-L, Wang G-D, Zhang L-L, Zulu L, Liu Y-H, Zheng Y-L, Rao Q. 2022. Sublethal effects of three insecticides on development and reproduction of Spodoptera frugiperda (Lepidoptera: noctuidae). Agronomy (Basel). 12(6):1334. https://doi.org/10.3390/agronomy12061334. [ Links ]
Xu L, Zhao C-Q, Zhang Y-N, Liu Y, Gu Z-Y. 2016. Lethal and sublethal effects of sulfoxaflor on the small brown planthopper Laodelphax striatellus. Journal of Asia-Pacific Entomology. 19(3):683-689. https://doi.org/10.1016/j.aspen.2016.06.013. [ Links ]
Yuan H-B, Li J-H, Liu Y-Q, Cui L, Lu Y-H, Xu X-Y, Li Z, Wu K-M, Desneux N. 2017. Lethal, sublethal and transgenerational effects of the novel chiral neonicotinoid pesticide cycloxaprid on demographic and behavioral traits of Aphis gossypii (Hemiptera: aphididae). Insect Science. 24(5):743-752. https://doi.org/10.1111/1744-7917.12357. [ Links ]
Zeng X, He Y, Wu J, Tang Y, Gu J, Ding W, Zhang Y. 2016. Sublethal effects of cyantraniliprole and imidacloprid on feeding behavior and life table parameters of Myzus persicae (Hemiptera: aphididae). Journal of Economic Entomology. 109(4):1595-1602. https://doi.org/10.1093/jee/tow104. [ Links ]
Zhou C, Liu L, Yang H, Wang Z, Long G-y, Jin D. 2017. Sublethal effects of imidacloprid on the development, reproduction, and susceptibility of the white-backed planthopper, Sogatella furcifera (Hemiptera: delphacidae). Journal of Asia-Pacific Entomology. 20(3):996-1000. https://doi.org/10.1016/j.aspen.2017.07.002. [ Links ]
Zuo Y-H, Chen M-E. 2014. Differential gene expression in male and female fat body in the oriental fruit fly, Bactrocera dorsalis. Archives of Insect Biochemistry and Physiology. 85(1):48-59. https://doi.org/10.1002/arch.21142. [ Links ]
 Correspondence:
Correspondence:
Gerda Fourie
Email: gerda1.fourie@fabi.up.ac.za
Received: 9 October 2023
Accepted: 26 February 2024













