Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Bioethics and Law
versión On-line ISSN 1999-7639
SAJBL vol.17 no.1 Cape Town mar. 2024
http://dx.doi.org/10.7196/SAJBL.2024.v17i1.2071
RESEARCH
Navigating ethical challenges of integrating genomic medicine into clinical practice: Maximising beneficence in precision oncology
M J KotzeI; K A GrantII; N C van der MerweIII; N W BarsdorfIV; M KrugerV
IBSc, BSc (Hons), MSc, PhD. Division of Chemical Pathology, Department of Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University; and the National Health Laboratory Service, Tygerberg Hospital, Cape Town, South Africa
IIMTech, PhD. Department of Biomedical Sciences, Faculty of Health and Wellness, Cape Peninsula University of Technology, Cape Town, South Africa
IIIBSc Agric, BSc (Hons), MSc, PhD; Division of Human Genetics, School of Pathology, Faculty of Health Sciences, University of the Free State, South Africa; and the National Health Laboratory Service, Universitas Hospital, Bloemfontein, South Africa
IVMHS, PhD; Office of Research Integrity and Ethics, Division for Research Development, Stellenbosch University, Stellenbosch, South Africa
VMB ChB, MMed Paed, FCP (SA), MPhil (Applied Ethics), PhD; Department of Paediatrics and Child Health and Human Research Ethics Committee 2, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
The development of gene expression profiling and next-generation sequencing technologies have steered oncogenomics to the forefront of precision medicine. This created a need for harmonious cooperation between clinicians and researchers to increase access to precision oncology, despite multiple implementation challenges being encountered. The aim is to apply personalised treatment strategies early in cancer management, targeting tumour subtypes and actionable gene variants within the individual's broader clinical risk profile and wellbeing. A knowledge-generating database linked to the South African Medical Research Council's Genomic Centre has been created for the application of personalised medicine, using an integrated service and research approach. Insights gained from patient experiences related to tumour heterogeneity, access to targeted therapies and incidental findings of pathogenic germline variants in tumour DNA, provided practice-changing evidence for the implementation of a cost-minimisation pathology-supported genetic testing strategy. Integrating clinical care with genomic research through data sharing advances personalised medicine and maximises precision oncology benefits.
The era of genomic medicine has witnessed major developments over the last decade, especially in the field of cancer genetics.[1] With the implementation of a new precision oncology scale for clinical actionability of molecular targets as proposed by the European Society of Medical Oncology in 2018, the utilisation of next-generation sequencing (NGS) technologies has become an integral part of cancer risk management.[2] The clinical utility of therapy targeted to genomic alterations stretches beyond immunohistochemistry (IHC)-based personalised medicine,[3] as demonstrated by significantly improved survival outcomes.[2] Although all malignancies are currently treated according to tumour type and/or stage, the gene expression information obtained from IHC can be enriched by NGS to increase the precision of the therapeutic approach. The genetic basis for differences in patients' response to therapy involves diverse signalling pathways, deficiency in the DNA double-strand break repair pathway, microsatellite instability and hypermutated tumour status.[4] Maximising beneficence in this context is challenging as every situation is unique.[5,6]
The use of molecular markers specific to each patient's cancer raised the expectation that targeted therapies could be administered independent of the underlying tumour histology.[7] However, the effectiveness of novel cancer-agnostic therapies is influenced by tumour heterogeneity and time-dependent molecular evolution, posing significant hurdles in the shift to genomically informed treatment.[8] While some cells respond to anti-cancer medication, others develop resistance and antagonistic effects fostering increased toxicity and tumour growth.[9] The interpretation of tumour-only NGS for the selection of targeted therapies is complicated by the presence of deleterious genetic variants in normal tissue and non-malignant conditions, adding to the liability risks for laboratories and healthcare practitioners.[10] New multi-cancer early detection blood tests that have entered the genomics landscape with the promise to improve the survival rate and reduce the costs associated with late-stage cancer treatments,[11] require real-world efficacy studies in relation to existing oncology practices.
The infrastructure and support systems required to bridge remaining clinical implementation gaps, address healthcare disparities and align incidental findings of germline pathogenic variants in tumour DNA with concurrent familial risk, are limited in Africa.[12,13] The lack of a healthcare model designed to characterise and gain a better understanding of these requirements resulted in the development of a pathology supported genetic testing (PSGT) platform,[14] enabling the application of personalised medicine through integrating service and research (PM-ISR). This approach provides a means to balance societal interest in the advancement of generalised knowledge v. individual benefit. Okunola et al.[15] accomplished this goal by using the PSGT platform for the return of research results to eligible South African (SA) patients with breast cancer participating in a pharmacogenetics study. Data sharing across diverse healthcare domains provided the opportunity to bridge the disconnect between inherited breast cancer caused by pathogenic BRCA1/2 variants and vitamin D- or aromatase inhibitor-related osteoporosis risk. In the past, analysis of SA patients for the two major cancer susceptibility genes, BRCA1 and BRCA2, was focused on surgical decision-making and to inform cascade family screening.[16] Today, the detection of pathogenic variants in the same genes can also serve as therapeutic targets for a new class of drugs called Poly (ADP-ribose) polymerase (PARP) inhibitors.[17] The finding that both germline and somatic BRCA1/2 variants respond similarly to PARP inhibitors ushered in a new era of personalised medicine where the boundaries between germline and tumour genetics became blurred. This introduced new ethical challenges such as the detection of variants of uncertain clinical significance (VUS) and associated complexities in communicating meaningful laboratory results.
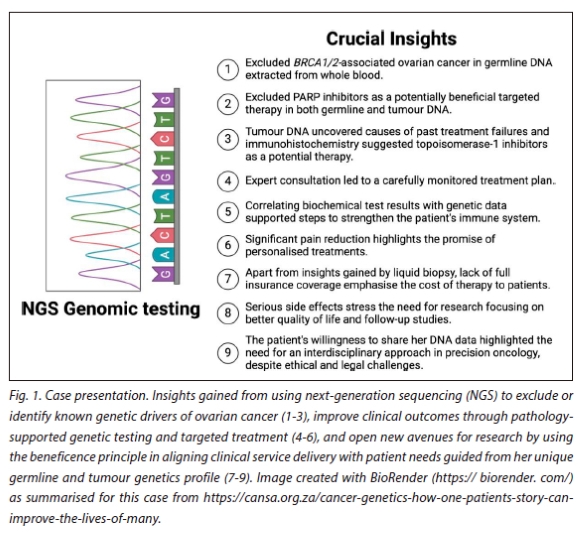
Beneficence in biomedical ethics as the central theme of this paper, relates to the obligation of healthcare professionals to ensure actions that collectively translate into benefit for patients. During the development of the multi-pronged PSGT platform for PM-ISR alongside standard pathology, experiences and uncertainties expressed by patients regarding shared clinical decision-making were noted. Insights gained from this perspective called for careful consideration of ethical duties continuously shaped by the depth of scrutiny applied by service providers. Fig. 1 illustrates an early example of precision oncology applied in a patient with metastatic ovarian cancer, who inspired the Open Genome Project as an NGS report-generating interface for clinical decisions beyond a single research objective.[12] Many learning points have been derived from this initiative, confirming clinically relevant relationships between tumour biomarkers and treatment responses on a case by case basis, using NGS technology combined with IHC.[15,18] The favourable impact of pharmacogenomic-derived recommendations on cancer progression supports the return of research results to individual patients. To prevent therapeutic misconception, the service and research arms of PSGT are guided by valid informed consent after careful consideration of previous pathology test results to help inform the appropriateness of NGS and the use of targeted therapies. Therefore, it is crucial to define when and how genomic assays add value to standard pathology in an ongoing manner. In the present study, we provide an overview of PM-ISR applied towards bridging remaining knowledge gaps in molecular pathology. We address healthcare disparities involving access to genomic assays for the selection of targeted therapies and align service delivery with patient needs while striving to balance conflicting ethical principles relevant to precision oncology.
Balancing conflicting ethical principles in precision oncology
Genomic research in clinical practice poses ethical challenges for the implementation of precision oncology, emphasising the need to balance beneficence, particularly the utility principle, with autonomy and privacy concerns. Gupta et al.[19] reviewed many concerns raised when using oncogenic mutations for targeted therapy. Adapting study design given sample size limitations characteristic of personalised medicine approaches, including n-of-1 clinical trials and small cohort implementation studies, requires institutional approval to uphold societal values and justice with regulatory oversight. The interplay of these ethical principles and the extent to which results can be generalised for clinical application varies in different situations, urging a reflective consideration of potential controversies in cooperative clinical decision-making. This is of particular relevance when one ethics principle conflicts with another. In an example presented by Munyaradzi,[5] it was stated that beneficence might be prioritised over autonomy if the latter impedes the use of genomic data to increase access to personalised healthcare. Utility as a principle of beneficence in biomedical ethics requires health workers to carefully analyse, evaluate and promote actions that are likely to benefit patients and the public.
The intention to increase access to precision oncology was reflected in the early hypothesis by Grant et al.[20]exploring where 'additional information provided by a genomic test could fit into the context of the current clinicopathological prognostication of early-stage breast carcinoma'. Over the last decade, this research question has been answered by practice-changing evidence,[21,22] with the use of the dynamic PSGT research translation service.[14,23] While it was challenging to balance benefits to patients with conflicts of interest arising during the first health technology assessment (HTA) performed in 2009 by a SA medical insurer, the PM-ISR model developed because of this process opened new avenues for research focused on minimisation of healthcare costs.[24,25] Ethical analysis of PSGT by institutional review boards furthermore set new standards to protect patients and benefit the public, as evidenced by a transition from population risk stratification to personal utility in translational research projects.[15,26] This relates to the readiness checklist compiled by Jongeneel et al.[12] for the implementation of genomic medicine in Africa. Inter-university cooperation of professionals from different spheres of healthcare facilitates compliance with ethical and legal requirements as a critical step in translating advances in precision oncology into benefits for cancer patients.[27,28] Fear of invasive surgery and chemotherapy were found to be important reasons for delaying a cancer diagnosis, affecting the usefulness of genetic information in precision oncology.
A summary of pertinent ethical challenges being encountered in the application of precision oncology is provided in Table 1. With the increased merging of traditional boundaries between diagnosis and treatment in cancer management, a shared duty is assumed by members of the multi-disciplinary service delivery team. Defining authorisation levels aligned with interdependent roles in scientist training and clinician education programmes is crucial in achieving a shared goal and compliance with regulatory requirements.
Bridging knowledge gaps in molecular pathology applied in oncology practice
Despite the potential of multi-omics to identify targeted therapies, some patients may benefit more than others due to distinct genetic and molecular profiles within a single tumour.[29] This phenomenon has increased the ethical and legal challenges characteristic ofinterdisciplinary research that cuts across different healthcare domains. Medical scientists and clinicians share the responsibility for the introduction of clinically relevant genome-scale and targeted multi-gene NGS panels into the healthcare system, with the aim to transform standard cancer care into precision oncology. PM-ISR aims to accomplish this goal by turning challenges into opportunities for the implementation of innovations in clinical practice, in line with the approach used by the Implementing GeNomics In pracTicE (IGNITE) network.[30]
An important research question discussed earlier is whether NGS can be applied independently from standard pathology tests such as IHC. Conversely, IHC assessments of oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2) and the proliferation marker (Ki67) status, can only approximate the Luminal A, Luminal B, HER2-enriched and basal breast cancer intrinsic subtypes.[31] Studies conducted in SA support the use of IHC in combination with a the transcriptional profiling of 150 genes (MammaPrint/BluePrint®) to accurately classify patients with hormone receptor-positive early-stage (1 and 2) invasive breast carcinoma, with up to three positive lymph nodes, into different treatment groups.[22,32] Despite the highest level 1A evidence for accurate prognosis and prediction of chemotherapy benefit (yes/no) based on the 70-gene MammaPrint® test used in SA from 2006, and the availability of NGS tests such as OncoDEEP® from 2014 for advanced solid tumours as applied in Fig. 1 and to predict response to immunotherapy, are only available within the private healthcare system of SA. These genomics access disparities relate to the two-tier healthcare system in SA, affecting not only the optimal use of anti-HER2 targeted therapy, endocrine and chemotherapy but also the potential to prevent a second primary cancer in patients with hormone receptor-positive BRCA1/2-associated breast cancer.[21,22,24] Further motivated by the high false-negative rate of HER2 status described earlier by Dabbs et al.[331 performed as part of a a 21-gene quantitative reverse transcription polymerase chain reaction (RT-PCR) assay (Oncotype DX®) in comparison with standard IHC and fluorescence in situ hybridisation (FISH), a research waiver of informed consent was granted for a quality assurance audit in SA.[20-221 This was in line with the SA Protection of Personal Information Act (POPIA), as specimens undergoing standard IHC and FISH testing were used in comparative microarray analysis for the same purpose. Reflex testing of tumours with discrepant IHC results,[21,22] provided an excellent example of turning challenges into opportunities, as pathology laboratories in SA started to add the HER2 copy number together with the ratio determined by FISH in their laboratory reports from around 2014.[32] As Oncotype DX® was designed to guide chemotherapy decision-making and not anti-HER2 therapy, ongoing comparative studies as reported by Bai et al..,[34] remain important with the recent transfer of the 150-gene RNA-based microarray to the MammaPrint/BluePrint® NGS test kit to increase access.
Addressing disparities in access to genomic assays for the selection of targeted therapies
Ethics guidelines generally reflect a limited focus on ways to increase access to targeted therapies, such as Herceptin (Trastuzumab) and PARP inhibitors in Africa, with oncology clinical trials including genomic tests perceived as complex and requiring expensive technologies. Maketha et al.[35] raised distributive justice concerns regarding BRCA1/2 founder/recurrent variant testing in SA, stemming from the historical interpretation of associated ancestral lineages as markers of race or ethnicity, leading to the direction of cost-effective genetic testing to specific population groups that benefited more than others. This aspect was addressed in the design of the rapid BRCA point-of-care (POC) Research Assay evaluated by Mampunye et al.,[24] which is applicable across the major ethnic groups in SA irrespective of age, family history or tumour type. The study cohort included a deceased patient diagnosed with invasive breast carcinoma of no special type, who tested positive for the pathogenic BRCA2 c.7934delG variant in DNA extracted from her bladder tissue using tumour-NGS, 4 years after receiving a low-risk breast cancer recurrence score based on transcriptional gene profiling. The possibility of breast cancer metastasis due to the potential misclassification of the tumour's molecular subtype was ruled out by the histopathology report indicating a new primary cancer in the bladder, not breast cancer metastasis. Confirmation of BRCA2 c.7934delG in the patient's germline DNA,[24] exposed a missed opportunity for the prevention of a second primary cancer caused by the most common BRCA1/2 founder variant previously identified in the SA population.[26]
Although the above-mentioned patient did not fulfil the criteria for BRCA1/2 testing at initial diagnosis of early breast cancer based on her age and family history, these genes were included upon the request of the treating oncologist when her tumour-NGS was performed as part of the OncoDEEP® precision oncology service. Effective communication between the clinician and laboratory scientists led to the integration of prior knowledge on the potential clinical implications for invasive urothelial carcinoma of the bladder,[36,37] into the dynamic PSGT process. Previously, only ovarian and triple-negative breast cancers were prioritised for tumour NGS including the BRCA1/2 genes, but the detection of a SA founder variant supported the broadening of the laboratory protocol and reporting of BRCA2 c.7934delG as an incidental finding in 2016. In this case, the identification of targeted therapies was the primary purpose of tumour-NGS and not to assess the patient's germline genetic status, as confirmed for BRCA2 c.7934delG by Sanger sequencing performed in SA. By pinpointing this pathogenic BRCA2 variant as a rare cause of metachronous breast and bladder cancer in the SA context, Mampunye et al.[24] initiated a process to intentionally report pathogenic BRCA/other germline variants in tumour DNA as secondary rather than incidental findings. This vision for the future of translational pharmacogenomics implies that any patient eligible for tumour genomic testing and their at-risk family members could benefit from rapid germline testing for confirmation of the common SA BRCA1/2 founder variants covered in the BRCA POC assay. A potential clinical dilemma caused by incidental findings can therefore be turned around to also benefit other breast cancer patients in future. For example, patients with early-stage Luminal A breast cancer may avoid chemotherapy despite the detection of a pathogenic BRCA1/2 variant in germline DNA. This finding relates to the design of the 150-gene microarray/NGS assay that includes all 10 cancer hallmarks,[38] in contrast to the less comprehensive 21-gene RT-PCR assay found to be inaccurate in patients of African descent with hormone-dependent breast cancer.[39] The significant racial/ethnic disparity in survival shown for all three Oncotype DX® breast cancer recurrence risk groups (low, intermediate and high), highlighted the need for recalibration of these scores. However, if we hope to move away from using race as a proxy for the root cause of cancer development or recurrence, given the availability of other similarly priced precision oncology solutions designed for the same purpose, providing different scores by race would be a step backward.
In a survey performed among members of the Southern African Society for Human Genetics (SASHG), most respondents agreed that geneticists and clinicians should refrain from using labels such as 'black' or 'white' as far as possible since these phenotypic descriptors do not reflect the complexity of populations from the African continent.[40] This survey was prompted by continued controversy highlighted at the 'Moving beyond race' panel discussion at the 2019 SASHG conference. The congress organising committee, after reviewing the submitted congress abstracts and discussion with bioethicists, allowed potential presenters to reconsider whether their usage of race, ethnicity or population group labels was specifically relevant to the respective studies and whether any population group has been unfairly excluded or targeted. Positive outcomes included revision and retraction of offending abstracts as received from different geographic regions in SA, indicating that the issue identified was not localised to a single area or institution. It marked a significant milestone in transitioning away from racial to clinicopathological interpretations, strengthening the respect for persons in low to middle-income countries who are often denied access to clinical trials due to cost and other disparities.[41]
With the successful implementation of NGS using the Oncomine™ BRCA Expanded Research Assay validated by the National Health Laboratory Service laboratory (NHLS) for routine use,[26] cancer patients are served in a manner that reflects the diverse SA demographics and population structure. Genetic testing in the public sector is funded by the Department of Health, with the patient being accountable for only a small fraction of the costs, if any. The currently used multigene NGS panel revealed a mutation-positive rate of approximately 15% in the patients studied, of which 25% are pathogenic founder/recurrent variants covered in the BRCA POC assay. To increase equitable access to targeted therapy in future amid ever-evolving NGS technologies,[26] BRCA1/2 POC testing combined with genomic counselling may provide the optimal starting point for breast, ovarian, prostate and pancreas cancer patients who are all eligible for PARP inhibitors.[17] Although the benefits outweigh the risks, targeting the repair of damaged DNA may cause serious side effects in some patients, ranging from temporary tiredness and breathlessness due to alterations in the number of blood cells to an increased risk of infection and bleeding problems that need to be managed as part of cancer care.
Aligning service delivery with patient needs through data sharing
Several tumour-based NGS applications have become commercially available in SA over the last decade including OncoDEEP® which currently targets more than 600 genes combined with IHC and other advanced technologies for shared decision-making. The use of this approach described by Laes et a/.[42], identified the KRAS (c.35 G>A, p.G12D, rs121913529) variant in the ovarian cancer patient (Fig. 1). Although no targeted therapy was available for any KRAS-associated cancer type at the time of testing, the Food and Drug Administration granted accelerated approval to Sotorasib (Lumakras™, Amgen Inc., USA) for patients with non-small cell lung cancer, in May 2021. Apart from the potential unavailability of targeted therapy informed by NGS, variants can be missed if the gene regions spanning genetic alterations of interest are not included in the platform at the time of the analysis. This was demonstrated in a comparative study of two different diagnostic NGS platforms, with a discrepancy noted for KRAS and ERBB2.[43] The divergent results also raised the question of whether gene variants identified in tumour DNA, in the absence of confirmed protein expression of the relevant biomarkers, might be misleading. Stage 4 solid tumours with progression on at least one line of standard therapy or when there is no standard of care for the type of cancer diagnosed, were considered the most appropriate criteria for tumour NGS.[43] However, failing to act within a specific turn-around time referred to as the 'window of opportunity' may result in a waste of resources.
In SA, delays in the utilisation of germline or tumour NGS and treatment initiation based on the results cause much distress in families. Disappointment expressed by patients due to 'not knowing' earlier (at first diagnosis of cancer) about a precision oncology option is difficult to manage. This problem recently escalated to the extent that two different NGS tests have been requested from different service providers in SA, nearly 3 years after the original diagnosis of metastatic colorectal cancer. When this delay was questioned by the patient, the 'cost of the test and likelihood of a non-treatable result they may not want' were given as reasons. This was upsetting to the patient as the cost of NGS combined with IHC was considered minimal compared with the medical aid co-payments made for failed non-targeted therapies. Expert opinion furthermore differed regarding the feasibility of obtaining a new biopsy from the metastatic tumour for NGS instead of using the stored primary tumour collected at first diagnosis and when the results became available consensus could not be reached regarding the optimal treatment plan. This scenario represents a lost opportunity for shared decision-making, despite access to both NGS reports with a minor discrepancy (possibly due to tumour heterogeneity) that did not affect the patient's treatment. By equipping oncologists with expert-led knowledge derived from NGS combined with IHC, well-informed clinical decisions can be made with greater confidence.[18]
Current advances in molecular pathology, especially for risk management of non-communicable diseases (NCDs) such as breast cancer with a high incidence worldwide, need to be leveraged to address concerns of late diagnosis at advanced stages (3 and 4) in Africa.[44-46] This may partly be explained by transport problems, poor healthcare literacy, fear of missing work due to appointments and fear of invasive surgery and chemotherapy side effects. However, increased awareness about the availability of clinically validated genomic assays where the costs are covered by medical schemes and/or state hospitals may increase the identification of early-stage breast cancer, enabling less invasive surgery and safe avoidance of chemotherapy in more patients.[28] Failure to inform patients about these genomic solutions based on the assumption that they will be unaffordable is considered unethical and legally unjustified, as a referral for a genetic/genomic test has implications for other family members who may pool resources to cover the cost.
Accurate estimation of cancer risk in families of deceased patients with deleterious variants in high-risk genes such as BRCA1/2 and TP53 known to contribute causally to tumour type, may benefit from stored germline DNA for follow-up testing in at-risk family members. To allow extended genetic testing of DNA from deceased cancer patients to the benefit of the extended family, Moremi et al.[47]applied PM-ISR for the integration of germline and tumour genetics across the illness and wellness domains. Evaluation of a multiplex NCD-pharmacogenetics assay in postmenopausal women with hormone receptor-positive breast cancer previously assessed for vitamin D and homocysteine levels as part of a biochemistry test panel,[15] provided supporting evidence to position POC genomics at the interface of familial, lifestyle and therapy-induced risk assessment in future.[48] Although screening for genetic underpinnings of lifestyle- or treatment-related comorbidities is not the primary target for genomic testing in cancer patients, secondary findings change how each individual is supported in their efforts to live healthier after diagnosis and treatment. Longitudinal research provides a comprehensive perspective on the trends, causal relationships and the impact of both external and intrinsic factors on the evolution of cancer genomics and DNA methylation over an extended timeframe. As traditional research ethics principles aimed at the protection of individual study participants are evolving towards serving the interests of society at large, Vos et al.[49] provided a compelling argument for a collaborative approach towards collective knowledge application.
Efficient collaboration between healthcare practitioners and laboratory scientists is imperative to enhance clinical access in the realm of precision oncology, notwithstanding the numerous challenges that may arise during its implementation. Fig. 2 shows the proposed NGS workflow for PM-ISR, from obtaining informed consent for first tier genotyping elevated to multi-gene panel testing and whole exome/genome sequencing in uninformative cases. Challenges encountered during the integration of research and service delivery are addressed by appropriate test selection aided by genetic counselling to facilitate clinical decision-support, elevation to extended genetic testing in uninformative cases and clinical implementation of risk reduction strategies tailored to the needs of the individual.[15] An umbrella informed consent document, refined over more than a decade, is completed together with a structured report-generating questionnaire to help clarify the benefits and limitations of PSGT as a novel approach to integrating clinical service delivery with translational genomic research. Moving from paper-based to electronic informed consent for genetic testing of NCDs was justified by the emergence of the coronavirus disease in 2019,[50] to enable a phased research translation process encouraging freedom to consent or decline testing without coercion. Understanding the risks of participation in translational research that may incorporate aspects of infectious diseases' host genetics, thus represents the first step in collecting personal information for the generation of adaptable feedback reports in the form of a case presentation for each patient, reflecting the collective knowledge of the researchers and healthcare practitioners involved in the research translation process. This is the purpose of the PSGT strategy: shifting the focus from volume of services to quality of care that prioritises early interventions and enhancement of individual patient experiences.[51-53]

Conclusion
The development of a framework for ethical decision-making started with the identification of important issues to address in precision oncology, followed by fact-finding as undertaken by the Cancer Association of SA for the MammaPrint® service.[54] This provided the first example of maximising beneficence in precision oncology in SA, based on solid scientific evidence from locally generated tumour genomics data incorporated into a new care pathway. PM-ISR enabled clinical, scientific, ethical and legal challenges encountered in pursuing precision oncology to be addressed as a shared responsibility at the intersection of participating laboratories and clinical practice. PSGT incorporating rapid POC testing for utilisation in remote areas to promote inclusivity of precision medicine will be a vital step towards achieving equitable cancer care in SA. This requires the development of a practical quality control plan incorporating patient-reported outcomes to determine the long-term impact of precision oncology treatments.
Declaration. The case study examples relate to projects N09/06/166 and N09/08/224 approved by the Human Research Ethics Committee of Stellenbosch University. Names of commercially available precision oncology tests previously used in comparative studies performed in SA were approved for inclusion in this article (HREC2-2024-5190).
Acknowledgements. This article is dedicated to the patient with colorectal cancer featured for the first time in this article, who died on the 22nd of March 2024, six months after receiving the results of two tumour-NGS tests performed for the same purpose. We thank Dr Dionne van Reenen of the University of the Free State for family support as well as all cancer patients and treating clinicians for sharing their experiences and relevant data to enable the preparation of this manuscript. Our gratitude goes to Dr Nicole van der Merwe for genetic counselling as part of the Open Genome Project, which supported the Master's and Doctoral degrees awarded to Mr Lwando Mampunye and Dr Abisola Okunola in 2020. We thank Drs Chantelle Scott, Daniel Olivier and Elouise Kroon of Stellenbosch University for reflecting on the outcome of actions taken within an evolving laboratory workflow currently applied in COVID-19 host genomics research. The figures included in this manuscript were constructed using BioRender and stock MS Word images, while parts of the text were shortened and rephrased with the assistance of the ChatGPT language model.
Author contributions. MJK conceptualised the idea of an inter-institutional knowledge-generating database enabling this overview of selected research translation studies performed by KG and NM over more than a decade, accompanied by ethical oversight of collaborative projects by NB and MK. NB wrote a first draft incorporated into this manuscript based on an Applied Genetics Workshop presentation on 30 October 2015, entitled 'A combined service and research approach: Maximising beneficence in the application of personalised medicine'. MK framed the current paper towards addressing the ethical issues arising from recent advances in NGS technologies. The last two authors, NB and MK, contributed equally to this study finally approved for publication.
Funding. The research reported in this publication was partly supported by the South African Medical Research Council with funds received from the National Department of Health (grant code 96847). The content and illustrations are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the SAMRC or National Department of Health. MJK received speaker fees from Roche Diagnostics (Pty) Ltd. used to cover the publication fees.
Conflicts of interest. MJK is a non-executive director and shareholder of Gknowmix (Pty) Ltd. to enable the PM-ISR approach described in this article as Part I of a scientist training and clinician education program. She serves on the Industry Expert Panel for Precision Oncology, which provided insights expressed in this article without any direct influence on the content from the sponsor, Roche Diagnostics (Pty) Ltd.
References
1. Horak P, Fröhling S, Glimm H. Integrating next-generation sequencing into clinical oncology: strategies, promises and pitfalls. ESMO Open 2016;1(5):1-11. https://doi.org/10.1136/esmoopen-2016-000094 [ Links ]
2. Martin-Romano P, Mezquita L, Hollebecque A, et al. Implementing the European Society for Medical Oncology scale for clinical actionability of molecular targets in a comprehensive profiling program: Impact on precision medicine oncology. JCO Precis Oncol 2022;6:1-11. https://doi.org/10.1200/PO.21.00484 [ Links ]
3. McCourt CM, Boyle D, James J, Salto-Tellez M. Immunohistochemistry in the era of personalised medicine. J Clin Pathol 2013;66(1):58-61. https://doi.org/10.1136/jclinpath-2012-201140 [ Links ]
4. Nagahashi M, Shimada Y, Ichikawa H, et al. Next-generation sequencing-based gene panel tests for the management of solid tumors. Cancer Sci. 2019;110(1):6-15. https://doi.org/10.1111/cas.13837 [ Links ]
5. Munyaradzi M. Critical reflections on the principle of beneficence in biomedicine. Pan Afr Med J 2012;11:29. [ Links ]
6. Mateo J, Steuten L, Aftimos P, et al. Delivering precision oncology to patients with cancer. Nat Med 2022;28:658-665. https://doi.org/10.1038/s41591-022-01717-2 [ Links ]
7. Mansinho A, Fernandes RM, Carneiro AV. Histology-agnostic drugs: a paradigm shift- a narrative review. Adv Ther 2023;40(4):1379-1392. https://doi.org/10.1007/s12325-022-02362-4 [ Links ]
8. Riedl JM, Moik F, Esterl T, Kostmann SM, Gerger A, Jost PJ. Molecular diagnostics tailoring personalised cancer therapy-an oncologist's view. Virchows Arch. 2023;Nov 20. https://doi.org/10.1007/s00428-023-03702-7
9. Sarkar S, Deyoung T, Ressler H, Chandler W. Brain tumors: development, drug resistance, and sensitisation - An epigenetic approach. Epigen 2023;18(1):2237761. https://doi.org/10.1080/15592294.2023.2237761 [ Links ]
10. Marchant G, Barnes M, Evans JP, LeRoy B, Wolf SM. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics 2020;48(1):11-43. https://doi.org/10.1177/1073110520916994 [ Links ]
11. Guerra CE, Sharma PV, Castillo BS. Multi-cancer early detection: The new frontier in cancer early detection. Annu Rev Med 2024;75:67-81. https://doi.org/10.1146/annurev-med-050522-033624 [ Links ]
12. Jongeneel CV, Kotze MJ, Bhaw-Luximon A, et al. A view on genomic medicine activities in Africa: implications for policy. Front Genet 2022;13:769919. https://doi.org/10.3389/fgene.2022.769919 [ Links ]
13. Owolabi P, Adam Y, Adebiyi E. Personalizing medicine in Africa: Current state, progress and challenges. Front Genet 2023;14:1233338. https://doi.org/10.3389/fgene.2023.1233338 [ Links ]
14. Kotze MJ, van Velden DP, Botha K, et al. Pathology-supported genetic testing directed at shared disease pathways for optimised health in later life. Pers Med 2013;10(5):497-507. https://doi.org/10.2217/pme.13.43 [ Links ]
15. Okunola AO, Baatjes KJ, Zemlin AE, et al. Pathology-supported genetic testing for the application of breast cancer pharmacodiagnostics: family counselling, lifestyle adjustments and change of medication. Expert Rev Mol Diagn 2023;23(5):431-443. https://doi.org/10.1080/14737159.2023.2203815
16. van der Merwe NC, Combrink HM, Ntaita KS, Oosthuizen J. Prevalence of clinically relevant germline BRCA variants in a large unselected South African breast and ovarian cancer cohort: a public sector experience. Front Genet 2022;13:834265. https://doi.org/10.3389/fgene.2022.834265 [ Links ]
17. Ragupathi A, Singh M, Perez AM, Zhang D. Targeting the BRCA1/2 deficient cancer with PARP inhibitors: Clinical outcomes and mechanistic insights. Front Cell Dev Biol 2023;11:1133472. https://doi.org/10.3389/fcell.2023.1133472 [ Links ]
18. Astras G, Papagiannopoulos CI, Kyritsis KA, Markitani C, Vizirianakis IS. Pharmacogenomic testing to guide personalised cancer medicine decisions in private oncology practice: a case study. Front Oncol 2020;28(10):521. https://doi.org/10.3389/fonc.2020.00521 [ Links ]
19. Gupta S, Smith TR, Broekman ML. Ethical considerations of neuro-oncology trial design in the era of precision medicine. J Neurooncol 2017;134(1):1-7. https://doi.org/10.1007/s11060-017-2502-0 [ Links ]
20. Grant KA, Apffelstaedt JP, Wright C, et al. MammaPrint Pre-screen Algorithm (MPA) reduces chemotherapy in patients with early-stage breast cancer. S Afr Med J 2013;3;103(8):522-526. https://doi.org/10.7196/samj.7223 [ Links ]
21. Grant KA, Pienaar FM, Brundyn K, et al. Incorporating microarray assessment of HER2 status in clinical practice supports individualised therapy in early-stage breast cancer. Breast 2015;24(2):137-142. https://doi.org/10.1016/j.breast.2014.12.006 [ Links ]
22. Grant KA, Myburgh EJ, Murray E, et al. Reclassification of early-stage breast cancer into treatment groups by combining the use of immunohistochemistry and microarray analysis. SA J Science 2019;115(3/4):51-56. https://doi.org/10.17159/sajs.2019/5461 [ Links ]
23. Kotze MJ. Gknowmix.com: A gateway to genomic healthcare. In: New Life Sciences: Future prospects: BioVisionAlexandria2010 / Bibliotheca Alexandrina; Editors, Ismail Serageldin, Ehsan Masood, with Mohamed El-Faham and Marwa El-Wakil. - Alexandria, Egypt: Bibliotheca Alexandrina, 2011, pp 435-443. https://www.bibalex.org/BiovisionAlexandria/Attachment/Attachment/file/BVA2010Web.pdf
24. Mampunye L, van der Merwe NC, Grant KA, et al. Pioneering BRCA1/2 point-of-care testing for integration of germline and tumor genetics in breast cancer risk management: A vision for the future of translational pharmacogenomics. Front Oncol 2021;29(11):619817. https://doi.org/10.3389/fonc.2021.619817 [ Links ]
25. Myburgh EJ, de Jager JJ, Murray E, Grant KA, Kotze MJ, de Klerk H. The cost impact of unselective vs selective MammaPrint testing in early-stage breast cancer in Southern Africa. Breast 2021;59:87-93. https://doi.org/10.1016/j.breast.2021.05.010 [ Links ]
26. van der Merwe NC, Ntaita KS, Stofberg H, Combrink HM, Oosthuizen J, Kotze MJ. Implementation of multigene panel testing for breast and ovarian cancer in South Africa: a step towards excellence in oncology for the public sector. Front Oncol 2023;12:938561. https://doi.org/10.3389/fonc.2022.938561 [ Links ]
27. Nembaware V, Johnston K, Diallo AA, et al. A framework for tiered informed consent for health genomic research in Africa. Nat Genet 2019;51(11):1566-1571. https://doi.org/10.1038/s41588-019-0520-x [ Links ]
28. Torrorey-Sawe R, van der Merwe N, Mining SK, Kotze MJ. Pioneering informed consent for return of research results to breast cancer patients facing barriers to implementation of genomic medicine: The Kenyan BRCA1/2 testing experience using whole exome sequencing. Front Genet 2020;11(170):1-6. https://doi.org/10.3389/fgene.2020.00170 [ Links ]
29. Ivanisevic T, Sewduth RN. Multi-omics integration for the design of novel therapies and the identification of novel biomarkers. Proteomes 2023;11(4):34. https://doi.org/10.3390/proteomes11040034 [ Links ]
30. Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics 2017;10(1):35. https://doi.org/10.1186/s12920-017-0273-2 [ Links ]
31. Pescia C, Guerini-Rocco E, Viale G, Fusco N. Advances in early breast cancer risk profiling: From histopathology to molecular technologies. Cancers (Basel) 2023;15(22):5430. https://doi.org/10.3390/cancers15225430 [ Links ]
32. Myburgh EJ, Langenhoven L, Grant KA, van der Merwe L, Kotze MJ. Clinical overestimation of HER2 positivity in early estrogen and progesterone receptor-positive breast cancer and the value of molecular subtyping using BluePrint. J Global Oncol 2016;16(3):314-322. https://doi.org/10.1200/JGO.2016.006072 [ Links ]
33. Dabbs DJ, Klein ME, Mohsin SK, Tubbs RR, Shuai Y, Bhargava R. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol 2011;29(32):4279e85. https://doi.org/10.1200/JCO.2011.34.7963 [ Links ]
34. Bai Q Lv H, Bao L, et al. Invasive breast cancer with HER2 >4.0 and <6.0: Risk classification and molecular typing by a 21-gene expression assay and MammaPrint plus BluePrint testing. Breast Cancer 2023;15:563-575. https://doi.org/10.2147/BCTT.S420738 [ Links ]
35. Makhetha M, Walters S, Aldous C. The review of genetic screening services and common BRCA1/2 variants among South African breast cancer patients. J Genet Couns 2023; Aug 1. https://doi.org/10.1002/jgc4.1755
36. Necchi A, Raggi D, Giannatempo P, et al. Exceptional response to olaparib in BRCA2-altered urothelial carcinoma after PD-L1 inhibitor and chemotherapy failure. Eur J Cancer 2018;(96):128-130. http://doi.org/10.1016/j.ejca.2018.03.021 [ Links ]
37. Kuang S, Li H, Feng J, Xu S, Le Y. Correlation of BRCA2 gene mutation and prognosis as well as variant genes in invasive urothelial carcinoma of the bladder. Cancer Biomark 2019;25(2):203-212. http://doi.org/10.3233/CBM-182379 [ Links ]
38. Haan JC, Bhaskaran R, Ellappalayam A, et al. MammaPrint and BluePrint comprehensively capture the cancer hallmarks in early-stage breast cancer patients. Genes Chromosom Cancer 2022;61(3):148-160. http://doi.org/10.1002/gcc.23014 [ Links ]
39. Hoskins KF, Danciu OC, Ko NY, Calip GS. Association of race/ethnicity and the 21-gene recurrence score with breast cancer-specific mortality among US women. JAMA Oncol 2021;7(3):370-378. http://doi.org/10.1001/jamaoncol.2020.7320 [ Links ]
40. Malope MF, da Rocha JEB, Fieggen KJ, as members of the SASHG Committee. Racial labelling in Human Genetics research in Southern Africa. https://sashg.org/wp-content/uploads/2021/07/Racial-labelling-survey_SASHG_10May21_website.pdf
41. Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open 2021;4(11):e2133205. http://doi.org/10.1001/jamanetworkopen.2021.33205 [ Links ]
42. Laes JF, Aftimos P, Barthelemy P, et al. The clinical impact of using complex molecular profiling strategies in routine oncology practice. Oncotarget 2018;9(29):20282-20293. http://doi.org/10.18632/oncotarget.24757 [ Links ]
43. Weiss GJ, Hoff BR, Whitehead RP, et al. Evaluation and comparison of two commercially available targeted next-generation sequencing platforms to assist oncology decision making. Onco Targets Ther 2015;8:959-967. https://doi.org/10.2147/OTT.S81995 [ Links ]
44. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a peri urban setting: a South African public hospital case series of over 1,000 women. Int J Cancer 2014;135(9):2173-2182. https://doi.org/10.1002/ijc.28861 [ Links ]
45. Espina C, McKenzie F, dos-Santos-Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann Epidemiol 2017;27(10):659-671. https://doi.org/10.1016/j.annepidem.2017.09.007 [ Links ]
46. Rayne S, Schnippel K, Kruger D, Benn CA, Firnhaber C. Delay to diagnosis and breast cancer stage in an urban South African breast clinic. S Afr Med J 2019;109(3):159-163. https://doi.org/10.7196/SAMJ.2019.v109i3.13283 [ Links ]
47. Moremi KE, Scott CJ, de Jager LJ, Pienaar R, Zemlin AE, Kotze MJ. Implementation of a pathology-supported genetic testing framework for return of research results to family members of deceased breast cancer patients with somatic TP53 variants. Breast 2021; 56(1):S64-S65. https://doi.org/10.1016/S0960-9776(21)00205-8 [ Links ]
48. Baatjes KJ, van der Merwe N, Moremi KE, et al. A rapid point-of-care test kit for improved clinical management of patients with breast cancer and associated co- morbidities: Significance of the MTHFR-homocysteine pathway. Breast 2023;68(1):S33-34. https://doi.org/10.1016/S0960-9776(23)00163-7 [ Links ]
49. Vos S, van Delden JJM, van Diest PJ, Bredenoord AL. Moral duties of genomics researchers: Why personalised medicine requires a collective approach. Trends Genet 2017;33:118-128. https://doi.org/10.1016/j.tig.2016.11.006 [ Links ]
50. Kotze MJ, van Rensburg SJ, Davids M, et al. Translating population risk into personal utility using a mobile phone app for application of genomic medicine integrating service and research in the COVID-19 era. Clin Chem Lab Med 2023;61(special suppl):S754, Abstract P0668. https://doi.org/10.1515/cclm-2023-7041 [ Links ]
51. van der Merwe N, Peeters AV, Pienaar FM, Bezuidenhout J, van Rensburg SJ, Kotze MJ. Exome Sequencing in a family with luminal-type breast cancer underpinned by variation in the methylation pathway. Int J Mol Sci 2017;18(2):467. https://doi.org/10.3390/ijms18020467 [ Links ]
52. Saelaert M, Mertes H, Moerenhout T, De Baere E, Devisch I. Ethical values supporting the disclosure of incidental and secondary findings in clinical genomic testing: a qualitative study. BMC Med Ethics 2020;21(1):9. https://doi.org/10.1186/s12910-020-0452-0 [ Links ]
53. Teisberg E, Wallace S, O'Hara S. Defining and implementing value-based health care: A strategic framework. Acad Med 2020;95(5):682-685. https://doi.org/10.1097/ACM.0000000000003122 [ Links ]
54. Herbst M C. Cancer Association of South Africa (CANSA): Fact Sheet on MammaPrint Test. https://cansa.org.za/files/2021/03/Fact-Sheet-on-Mammaprint-Test-March-2021.pdf.
 Correspondence:
Correspondence:
M J Kotze
maritha@sun.ac.za
Accepted 4 April 2024














