Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.3 Johannesburg Mar. 2015
GENERAL PAPERS
Enrichment of low-grade colemanite concentrate by Knelson Concentrator
T. UsluI; O. CelepI; M. SavașII
IDivision of Mineral & Coal Processing, Department of Mining Engineering, Karadeniz Technical University, Trabzon, Turkey
IIEmet Baron Works, Kütahya Turkey
SYNOPSIS
This study investigates the enrichment of a low-grade colemanite concentrate (-3 mm) using a Knelson centrifugal gravity concentrator. Due to its low boron content, the concentrate is unsaleable and has to be stored under appropriate conditions to avoid potential environmental problems. The low-grade colemanite concentrate was comminuted to size fractions of -1 mm, -0.5 mm, and -0.15 mm before treatment in the Knelson Concentrator. The effects of particle size, fluidizing water velocity, and bowl speed on the enrichment process were examined. The B2O3 content of the concentrate was increased from 33.96% to a maximum of 45.52%. B2O3 recovery increased with increasing bowl speed and particle size, and decreased with increasing fluidizing water velocity. The enrichment process also rejected arsenic and iron to some extent, with a maximum reduction of arsenic from 1360 g/t to 765 g/t and iron from 0.88% to 0.33%.
Keywords: boron, colemanite, gravity concentration, centrifugal concentration, Knelson Concentrator
Introduction
Turkey has the world's largest boron deposits, with 72% of the global resources (Uslu, 2007). The commercially most important boron minerals are borax (Na2B4O7.10H2O), colemanite (Ca2B6On.5H2O), and ulexite (NaCaB5O9.8H2O) (Christogerou et al., 2009). Colemanite is the most abundant boron mineral in the Turkish deposits (Koca, savas, and Koca, 2003; Yildiz, 2004). The major gangue minerals associated with colemanite ores are clays, carbonate minerals, and, to a less extent, arsenic minerals (Koca and Savaş, 2004). Colemanite ores are concentrated by attrition scrubbing followed by screening and classification to remove clay minerals (Koca and Savaş 2004; Uslu and Arol, 2004; Acarkan et al., 2005; Gül, Kaytaz, and Önal, 2006).
Colemanite deposits in Turkey are exploited by two sub-units of Eti Mine Works (Emet Boron Works, Bigadiç Boron Works). In the two concentrators of Emet Boron Works, approximately 1.5 Mt/a = of ore containing 25-28% B2O3 is processed to produce 700 kt of concentrate containing up to 36-42% B2O3. However, 70 kt/a of concentrate is stockpiled since it cannot be marketed or used in the production of boric acid due to its low grade. Approximately 600 kt of low-grade concentrate have already been accumulated in the stockpiles (EBW, 2014). Stockpiling of this concentrate brings about potential problems, including the occupation of large areas of land and the environmental pollution due to its exposure to atmospheric effects. Figure 1 shows a stockpile of low-grade colemanite concentrate in the area of Espey Mine. Treatment of this low-grade concentrate by a suitable method is important for resource efficiency and elimination of the problems associated with stockpiling.

The Knelson Concentrator is essentially a hindered settling device, related to the hydrosizer, with centrifugal force substituting for the force of gravity. It consists of a rotating ribbed cone (bowl) with fluidized concentrate retention zones between the ribs. Feed slurry enters through a central feed tube at the bottom of the cone and is thrown outwards by centrifugal force. Heavy (or large) particles are trapped in the retention zone between the ribs, while the light particles (or fine particles) are carried upward into the tailings stream by the slurry stream. Injection of water through small holes located in the retention zones promotes the formation of a fluidized and permeable concentrate bed consisting of heavier particles (Uslu, Sahinoglu, and Yavuz, 2012). Despite its wide range of applications, the utilization of the Knelson Concentrator for enrichment of boron minerals has not been previously reported. In the case of colemanite enrichment by the Knelson Concentrator, clay and other light or low specific gravity particles that are generally dispersed finely in the slurry would be removed from the bowl as overflow, while colemanite particles would remain in the bowl (Figure 2). Preliminary tests (Uslu, Celep, and Savas, 2012) demonstrated that the Knelson Concentrator can be used for enrichment of the low-grade colemanite concentrate. In this study, effects of various factors including particle size, bowl speed, and fluidizing water velocity on the enrichment process are investigated.

Materials and method
Materials
A sample of low-grade colemanite concentrate (-3 mm) was obtained from the Espey colemanite concentrator of Emet Boron Works. The Espey concentrator and sampling point are illustrated in Figure 3. The chemical analysis and particle size analysis of the sample are given in Table I and Figure 4, respectively.



As seen from Figure 4, 80% of the low-grade concentrate is <1.5 mm. The B2O3 grade is higher in coarse particle fractions due to greater amount of fine clay particles in the fine fractions.
Method
The sample was ground to three different size fractions (-1 mm, -0.5 mm, and -0.15 mm) in a rod mill. Each fraction was subjected to the enrichment process in a laboratory batch-type Knelson Concentrator (KC-MD3) (Figure 5). The effects of bowl speed [500 r/min (11.2 G-force), 1000 r/min, (45 G-force), 1500 r/min, (100 G-force), and 2000 r/min (179 G-force)] and fluidizing water velocity (1 L/min, 3 L/min, 5 L/min, and 7 L/min) were investigated. Feed pulp at approximately 10% solids by weight was prepared in a volume of 500 mL in a 1000 mL beaker. The beaker contents were agitated for 15 minutes using an IKA RW-20 type overhead stirrer equipped with a 45° pitched blade turbine (four blade, 50 mm in diameter). The dispersed slurry was fed to the Knelson Concentrator at a rate of 25 g/min. Overflow (tailings) was collected in a bucket while underflow (colemanite concentrate) remained in the bowl. The bowl contents (concentrate) were washed into beakers. After dewatering by using a vacuum filter, the products were dried, weighed, and analysed for boron oxide (B2O3), iron (Fe), and arsenic (As). Analyses were conducted in the laboratory of Emet Boron Works. The B2O3 recovery and Fe and As removal were calculated by using the following equations:
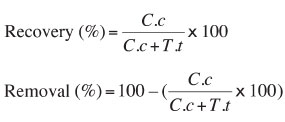

where C is amount of concentrate (g), c the grade of concentrate (%), Γ the amount of tailings (g), and t the grade of the tailings (%).
Results and discussion
In three of the total of 36 tests, no concentrate was produced and all of the feed reported to the tailings due to the interactive combination of fine particle size, low bowl speed, and high fluidizing water velocities. The remaining 33 tests were used for the evaluation of the results.
The B2O3recovery increased generally with increasing the bowl speed or decreasing fluidizing water velocity (Figure 6). The increase in B2O3recovery results from increasing centrifugal forces at high bowl speeds. Decreasing the velocity of fluidizing water resulted in lower B2O3grades at the same bowl speeds due to particles being rejected by fluidizing water. At the lowest particle size (-0.15 mm), fluidizing water flow had little effect on the concentrate grade, and grades were in general higher than those at coarser particle sizes. This is probably due to more complete liberation of colemanite at a finer grind, resulting in a purer concentrate, and more evenly-sized concentrate at finer grind with lesser susceptibility to changes in the fluidizing water flow rate.
The adverse effect of increasing bowl speed and decreasing water velocity on iron removal (Figure 7) can be explained in the same manner. The increased centrifugal force at high bowl speeds caused clay particles to be retained between the ribs, despite their fine sizes, i.e., fine and light particles were also affected by the centrifugal force. Higher fluidizing flow assists in removing clay but also adversely affects colemanite recovery. Since iron is associated with the clay minerals, iron removal is linked with the rejection of clay minerals in the tailings. Arsenic removal generally increased with decreasing bowl speed and increasing water velocity (Figure 8), following a similar trend as clay/iron.
Although a lower particle size affected the B2O3 recovery adversely, it had a positive effect on iron and arsenic removal (Figures 6-8). Size reduction generated considerable amounts of colemanite fines, together with liberated clay particles. In the enrichment process, fine colemanite particles, as well as the clays, were lost in the overflow as tailings. The enhancement of iron removal by size reduction is attributed to the improved liberation of iron-bearing clay minerals.



The minimum B2O3content for -3 mm concentrate in the boron market is 36%. On the other hand, only concentrates with a B2O3 content of >40% are used to produce boric acid in Emet Boron Works. In terms of resource efficiency, a B2O3 recovery exceeding 70% is considered to be acceptable in the plant. Test results and conditions that provided acceptable B2O3 recoveries (>70%) and B2O3 grades (>36%) are summarized in Table II. Although concentrates containing up to 45.52% B2O3 were produced after grinding to -0.5 mm, at the expense of high losses from the -3 mm concentrate, a B2O3 content of 40.2% could be produced at a recovery of 86.48%.
While up to 91.41% of the arsenic and up to 97.85% of the iron could be removed, arsenic removals of 1.36-22.66% and iron removals of 13.80-62.97% were achieved in tests that yielded acceptable B2O3 recoveries and grades. Iron and arsenic removals at optimum grade-recovery combination of B2O3 were 57.95% and 15.39%, respectively. The minimum arsenic and iron grades of the concentrates were 765 g/t and 0.33%, respectively.
High levels of arsenic removal were accompanied by a decrease in B2O3 recovery. This can be attributed to the finely disseminated nature of the arsenic, mainly as realgar in the colemanite concentrate. It is therefore extremely difficult to liberate arsenic minerals by size reduction, and the behaviour of arsenic was similar to that of boron in the concentration process. Size reduction to -0.15 mm allowed considerable arsenic removal at the expense of a sharp decrease in B2O3 recovery due to high boron losses as fines.
When the results of this study were compared to those of the preliminary study (Uslu, Celep, and Savas, 2012), in which low-grade concentrate (-3 mm) was processed directly in the Knelson Concentrator, without prior size reduction, it was found that size reduction of low-grade concentrate (-3 mm) did not result in a concentrate with higher B2O3 grade, due to the high of B2O3 losses to the tailings.
Only one study has been reported previously that resulted in enrichment of -3 mm low-grade colemenite concentrate of Emet Boron Works. The B2O3 grade was increased up to 49%, with recoveries over 80%, by grinding to -0.25 mm followed by ultrasonic pre-treatment and flotation (Ozkan and Gungoren, 2012).
Conclusions
The Knelson Concentrator was applied for the beneficiation of a low-grade colemanite concentrate. The B2O3 grade was increased from 33.96% to a maximum of 45.52%. However, optimum concentration was carried out by increasing the B2O3 grade to 40.2% at a recovery of 86.48%. A B2O3 grade of 41.88% at 78.85% recovery is another remarkable result. With the optimum concentration process, the iron content was reduced from 0.88% to 0.68%, and arsenic content from 1360 g/t to 1240 g/t. Reduction of low-grade colemanite (-3 mm) to finer sizes (-0.15 mm) did not enhance the enrichment process. The separation performance depended on the bowl speed and fluidizing water velocity, with a close interaction between these two parameters. The results show that the Knelson Concentrator is a promising candidate for producing marketable concentrates from low-grade colemanite concentrate.
The current study is the first to use a Knelson Concentrator for processing boron minerals. A large-scale Knelson Concentrator, such as Knelson KC-CVD, should be used in further studies due to its suitability for industrial mineral applications.
References
Acarkan, N., Bulut, G., Kangal, O., and Ónal, G. 2005. A new process for upgrading boron content and recovery of borax concentrate. Minerals Engineering, vol. 18. pp. 739-741. [ Links ]
Celep, O., Alp, I., Deveci, H., Vicil, M., and Yilmaz, T. 2008. Gold recovery from Mastra (Gümüshane) ore using Knelson Centrifugal Saparator. Istanbul Earth Sciences Review, vol. 19, no. 2. pp. 175-182. (In Turkish). [ Links ]
Christogerou, A., Kavas, T., Pontikes, Y., Koyas, S., Tabak, Y., and Angelopoulos, G.N. 2009. Use of boron wastes in the production of heavy clay ceramics. Ceramics International, vol. 35. pp. 447-452. [ Links ]
EBW (Eti Mine Works). 2014. Daily Work Report of Emet Boron Works, February 2014. [ Links ]
GüL, A., Kaytaz, Y., and Ónal, G. 2006. Beneficiation of colemanite tailings by attrition and flotation. Minerals Engineering, vol. 19. pp. 368-369. [ Links ]
Kawatra, S.K. and Eisele, T.C. 2001. Coal desulphurization, high-efficiency preparation methods. Taylor and Francis, New York. [ Links ]
Koca, S. and Savas, M. 2004. Contact angle measurements at the colemanite and realgar surfaces. Applied Surface Science, vol. 225. pp. 347-355. [ Links ]
Koca, S., Savas, M., and Koca, H. 2003. Flotation of colemanite from realgar. Minerals Engineering, vol. 16. pp. 479-482. [ Links ]
Ozkan,S. and Gungoren, C. 2012. Enhancement of colemanite flotation by ultrasonic pre-treatment. Physicochemical Problems of Mineral Processing, vol. 48, no. 2. pp. 455-462. [ Links ]
Uslu, T. and Arol, A.I. 2004. Use of boron waste as an additive in red bricks. Waste Management, vol. 24. pp. 217-220. [ Links ]
Uslu, T. 2007. Use of boron as energy source. Proceedings of the Sixth Energy Symposium of Turkey. Chamber of Electrical Engineers. pp. 433-450. [ Links ]
Uslu, T., Celep, O.E., and Savas, M. 2012. A preliminary research for upgrading of low grade colemanite concentrate by scrubbing and Knelson Concentrator. Proceedings of the 13th International Mineral Processing Symposium, Bodrum, Turkey, 10-12 October 2012. pp. 653-658 [ Links ]
Uslu, T., Sahinoglu, E., and Yavuz, M. 2012. Desuluphurization and deashing of oxidized fine coal by Knelson concentrator. Fuel Processing Technology, vol. 101. pp. 94-100. [ Links ]
Yildiz, O. 2004. The effect of heat treatment on colemanite processing: a ceramics application. Powder Technology, vol. 142. pp. 7-12. [ Links ]
Paper received Apr. 2014
Revised paper received Jul. 2014













